Modulation of intein activity by its neighboring extein substrates
- PMID: 19541659
- PMCID: PMC2708771
- DOI: 10.1073/pnas.0904366106
Modulation of intein activity by its neighboring extein substrates
Abstract
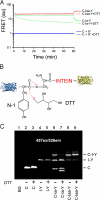
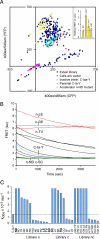
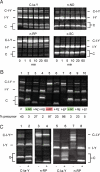
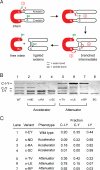
Inteins comprise a large family of phylogenetically widespread self-splicing protein catalysts that colonize diverse host proteins. The evolutionary and functional relationship between the intein and the split-host protein, the exteins, is largely unknown. To probe an association, we developed an in vivo and in vitro intein assay based on FRET. The FRET assay reports cleavage of the intein from its N-terminal extein. Applying this assay to randomized extein libraries, we show that the nature of the extein substrate bordering the intein can profoundly influence intein activity. Residues proximal to the intein-splicing junction in both N- and C-terminal exteins can accelerate the N-terminal cleavage rate by >4-fold or attenuate cleavage by 1,000-fold, both resulting in compromised self-splicing efficiency. The existence and the magnitude of extein effects require consideration for maximizing the utility of inteins in biotechnological applications, and they predict biases in intein integration sites in nature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gimble FS, Thorner J. Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae. Nature. 1992;357:301–306. - PubMed
-
- Belfort M, Derbyshire V, Stoddard B, Wood D. Inteins and Homing Endonucleases. Berlin: Springer; 2005.
-
- Pietrokovski S. Intein spread and extinction in evolution. Trends Genet. 2001;17:465–472. - PubMed
-
- Chong S, Williams KS, Wotkowicz C, Xu M-Q. Modulation of protein splicing of the Saccharomyces cerevisiae vacuolar membrane ATPase intein. J Biol Chem. 1998;273:10567–10577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

