Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma
- PMID: 19541710
- PMCID: PMC3471127
- DOI: 10.1183/09031936.00186508
Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma
Abstract
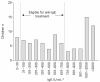
This study describes the clinical characteristics and corticosteroid responsiveness of children with difficult asthma (DA). We hypothesised that complete corticosteroid responsiveness (defined as improved symptoms, normal spirometry, normal exhaled nitric oxide fraction (F(eNO)) and no bronchodilator responsiveness (BDR <12%)) is uncommon in paediatric DA. We report on 102 children, mean+/-sd age 11.6+/-2.8 yrs, with DA in a cross-sectional study. 89 children underwent spirometry, BDR and F(eNO) before and after 2 weeks of systemic corticosteroids (corticosteroid response study). Bronchoscopy was performed after the corticosteroid trial. Of the 102 patients in the cross-sectional study, 88 (86%) were atopic, 60 (59%) were male and 52 (51%) had additional or alternative diagnoses. Out of the 81 patients in the corticosteroid response study, nine (11%) were complete responders. Of the 75 patients with symptom data available, 37 (49%) responded symptomatically, which was less likely if there were smokers in the home (OR 0.31, 95% CI 0.02-0.82). Of the 75 patients with available spirometry data, 35 (46%) had normal spirometry, with associations being BAL eosinophilia (OR 5.43, 95% CI 1.13-26.07) and high baseline forced expiratory volume in 1 s (FEV(1)) (OR 1.08, 95% CI 1.02-1.12). Of these 75 patients, BDR data were available in 64, of whom 36 (56%) had <12% BDR. F(eNO) data was available in 70 patients, of whom 53 (75%) had normal F(eNO). Airflow limitation data was available in 75 patients, of whom 17 (26%) had persistent airflow limitation, which was associated with low baseline FEV(1) (OR 0.93, 95% CI 0.90-0.97). Only 11% of DA children exhibited complete corticosteroid responsiveness. The rarity of complete corticosteroid responsiveness suggests alternative therapies are needed for children with DA.
Figures




References
-
- American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. - PubMed
-
- Lex C, Payne DN, Zacharasiewicz A, et al. Is a two-week trial of oral prednisolone predictive of target lung function in pediatric asthma? Pediatr Pulmonol. 2005;39:521–527. - PubMed
-
- Payne D, Bush A. Phenotype-specific treatment of difficult asthma in children. Paediatr Respir Rev. 2004;5:116–123. - PubMed
-
- Ranganathan SC, Payne DN, Jaffe A, et al. Difficult asthma: defining the problems. Pediatr Pulmonol. 2001;31:114–120. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
