The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary
- PMID: 19541765
- PMCID: PMC2736073
- DOI: 10.1210/en.2009-0206
The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary
Abstract
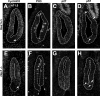
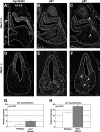
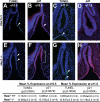
The pituitary is an endocrine gland responsible for the release of hormones, which regulate growth, metabolism, and reproduction. Diseases such as hypopituitarism or pituitary adenomas are able to disrupt pituitary function leading to suboptimal function of the entire endocrine system. Growth of the pituitary during development and adulthood is a tightly regulated process. Hairy and enhancer of split (HES1), a transcription factor whose expression is initiated by the Notch signaling pathway, is a repressor of cell cycle inhibitors. We hypothesize that with the loss of Hes1, pituitary progenitors are no longer maintained in a proliferative state, choosing instead to exit the cell cycle. To test this hypothesis, we examined the expression of cell cycle regulators in wild-type and Hes1-deficient pituitaries. Our studies indicate that in early pituitary development [embryonic day (e) 10.5], cells contained in the Rathke's pouch of Hes1 mutants have decreased proliferation, indicated by changes in phosphohistone H3 expression. Furthermore, pituitaries lacking Hes1 have increased cell cycle exit, shown by significant increases in the cyclin-dependent kinase inhibitors, p27 and p57, from e10.5 to e14.5. Additionally, Hes1 mutant pituitaries have ectopic expression of p21 in Rathke's pouch progenitors, an area coincident with increased cell death. These observations taken together indicate a role for HES1 in the control of cell cycle exit and in mediating the balance between proliferation and differentiation, allowing for the properly timed emergence of hormone secreting cell types.
Figures



Similar articles
-
Hes1 is required for pituitary growth and melanotrope specification.Dev Biol. 2007 Apr 15;304(2):455-66. doi: 10.1016/j.ydbio.2006.11.010. Epub 2006 Nov 10. Dev Biol. 2007. PMID: 17367776 Free PMC article.
-
p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors.Dev Biol. 2006 Oct 1;298(1):22-31. doi: 10.1016/j.ydbio.2006.05.036. Epub 2006 Jun 2. Dev Biol. 2006. PMID: 16899237
-
Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1.Dev Biol. 2009 Jan 1;325(1):151-61. doi: 10.1016/j.ydbio.2008.10.010. Epub 2008 Nov 1. Dev Biol. 2009. PMID: 18996108 Free PMC article.
-
Expression dynamics and functions of Hes factors in development and diseases.Curr Top Dev Biol. 2014;110:263-83. doi: 10.1016/B978-0-12-405943-6.00007-5. Curr Top Dev Biol. 2014. PMID: 25248479 Review.
-
Stem cells, differentiation and cell cycle control in pituitary.Front Horm Res. 2010;38:15-24. doi: 10.1159/000318490. Epub 2010 Jul 5. Front Horm Res. 2010. PMID: 20616491 Review.
Cited by
-
Genetic regulation of murine pituitary development.J Mol Endocrinol. 2015 Apr;54(2):R55-73. doi: 10.1530/JME-14-0237. Epub 2015 Jan 13. J Mol Endocrinol. 2015. PMID: 25587054 Free PMC article. Review.
-
Small-molecule screening yields a compound that inhibits the cancer-associated transcription factor Hes1 via the PHB2 chaperone.J Biol Chem. 2018 May 25;293(21):8285-8294. doi: 10.1074/jbc.RA118.002316. Epub 2018 Mar 9. J Biol Chem. 2018. PMID: 29523683 Free PMC article.
-
Expression of the PAX8/PPARγ Fusion Protein Is Associated with Decreased Neovascularization In Vivo: Impact on Tumorigenesis and Disease Prognosis.Genes Cancer. 2010 May;1(5):480-492. doi: 10.1177/1947601910373545. Genes Cancer. 2010. PMID: 20827445 Free PMC article.
-
Overexpression of the Notch3 receptor and its ligand Jagged1 in human clinically non-functioning pituitary adenomas.Oncol Lett. 2013 Mar;5(3):845-851. doi: 10.3892/ol.2013.1113. Epub 2013 Jan 7. Oncol Lett. 2013. PMID: 23426998 Free PMC article.
-
Whole Exome Sequencing in Patients With Ectopic Posterior Pituitary.J Endocr Soc. 2022 Aug 11;6(10):bvac116. doi: 10.1210/jendso/bvac116. eCollection 2022 Oct 1. J Endocr Soc. 2022. PMID: 36042976 Free PMC article.
References
-
- Burrows HL, Douglas KR, Seasholtz AF, Camper SA 1999 Genealogy of the anterior pituitary gland: tracing a family tree. Trends Endocrinol Metab 10:343–352 - PubMed
-
- Rizzoti K, Lovell-Badge R 2005 Early development of the pituitary gland: induction and shaping of Rathke’s pouch. Rev Endocr Metab Disord 6:161–172 - PubMed
-
- Zhu X, Rosenfeld MG 2004 Transcriptional control of precursor proliferation in the early phases of pituitary development. Curr Opin Genet Dev 14:567–574 - PubMed
-
- Treier M, O'Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG 2001 Hedgehog signaling is required for pituitary gland development. Development 128:377–386 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

