Conditional overexpression of connective tissue growth factor disrupts postnatal lung development
- PMID: 19541844
- PMCID: PMC2874441
- DOI: 10.1165/rcmb.2009-0068OC
Conditional overexpression of connective tissue growth factor disrupts postnatal lung development
Abstract
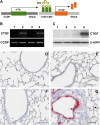
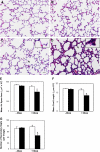
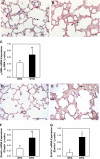
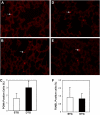
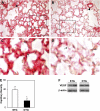
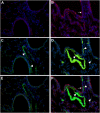
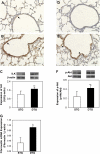
Connective tissue growth factor (CTGF) is a member of an emerging family of immediate-early gene products that coordinates complex biological processes during development, differentiation, and tissue repair. Overexpression of CTGF is associated with mechanical ventilation with high tidal volume and oxygen exposure in newborn lungs. However, the role of CTGF in postnatal lung development and remodeling is not well understood. In the present study, a double-transgenic mouse model was generated with doxycycline-inducible overexpression of CTGF in respiratory epithelial cells. Overexpression of CTGF from Postnatal Days 1-14 resulted in thicker alveolar septa and decreased secondary septal formation. This is correlated with increased myofibroblast differentiation and disorganized elastic fiber deposition in alveolar septa. Overexpression of CTGF also decreased alveolar capillary network formation. There were increased alpha-smooth muscle actin expression and collagen deposition, and dramatic thickening in the peribronchial/peribronchiolar and perivascular regions in the double-transgenic lungs. Furthermore, overexpression of CTGF increased integrin-linked kinase expression, activated its downstream signaling target, Akt, as well as increased mRNA expression of fibronectin. These data demonstrate that overexpression of CTGF disrupts alveologenesis and capillary formation, and induces fibrosis during the critical period of alveolar development. These histologic changes are similar to those observed in lungs of infants with bronchopulmonary dysplasia.
Figures


Similar articles
-
CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol. 2011 Mar;300(3):L330-40. doi: 10.1152/ajplung.00270.2010. Epub 2011 Jan 14. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21239535 Free PMC article.
-
Integrin-linked kinase mediates CTGF-induced epithelial to mesenchymal transition in alveolar type II epithelial cells.Pediatr Res. 2015 Apr;77(4):520-7. doi: 10.1038/pr.2015.8. Epub 2015 Jan 12. Pediatr Res. 2015. PMID: 25580742
-
Connective tissue growth factor stimulates the proliferation, migration and differentiation of lung fibroblasts during paraquat-induced pulmonary fibrosis.Mol Med Rep. 2015 Jul;12(1):1091-7. doi: 10.3892/mmr.2015.3537. Epub 2015 Mar 24. Mol Med Rep. 2015. PMID: 25815693 Free PMC article.
-
CTGF: A potential therapeutic target for Bronchopulmonary dysplasia.Eur J Pharmacol. 2019 Oct 5;860:172588. doi: 10.1016/j.ejphar.2019.172588. Epub 2019 Aug 1. Eur J Pharmacol. 2019. PMID: 31377154 Review.
-
Drugs targeting CTGF in the treatment of pulmonary fibrosis.J Cell Mol Med. 2024 May;28(10):e18448. doi: 10.1111/jcmm.18448. J Cell Mol Med. 2024. PMID: 38774993 Free PMC article. Review.
Cited by
-
Fibrosis of two: Epithelial cell-fibroblast interactions in pulmonary fibrosis.Biochim Biophys Acta. 2013 Jul;1832(7):911-21. doi: 10.1016/j.bbadis.2013.03.001. Epub 2013 Mar 14. Biochim Biophys Acta. 2013. PMID: 23499992 Free PMC article.
-
Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung.Respir Res. 2015 Jan 16;16(1):4. doi: 10.1186/s12931-014-0162-6. Respir Res. 2015. PMID: 25591994 Free PMC article.
-
Tracheal Aspirate Levels of the Matricellular Protein SPARC Predict Development of Bronchopulmonary Dysplasia.PLoS One. 2015 Dec 11;10(12):e0144122. doi: 10.1371/journal.pone.0144122. eCollection 2015. PLoS One. 2015. PMID: 26656750 Free PMC article.
-
A mouse strain where basal connective tissue growth factor gene expression can be switched from low to high.PLoS One. 2010 Sep 22;5(9):e12909. doi: 10.1371/journal.pone.0012909. PLoS One. 2010. PMID: 20877562 Free PMC article.
-
Patho-mechanisms of the origins of bronchopulmonary dysplasia.Mol Cell Pediatr. 2021 Dec 11;8(1):21. doi: 10.1186/s40348-021-00129-5. Mol Cell Pediatr. 2021. PMID: 34894313 Free PMC article. Review.
References
-
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. - PubMed
-
- Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in post surfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. - PubMed
-
- Coalson JJ, Winter VT, Delemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med 1995;152:640–646. - PubMed
-
- Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol 2006;30:179–184. - PubMed
-
- Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

