Modulation of the E2F1-driven cancer cell fate by the DNA damage response machinery and potential novel E2F1 targets in osteosarcomas
- PMID: 19541929
- PMCID: PMC2708823
- DOI: 10.2353/ajpath.2009.081160
Modulation of the E2F1-driven cancer cell fate by the DNA damage response machinery and potential novel E2F1 targets in osteosarcomas
Abstract
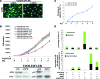
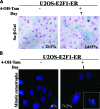
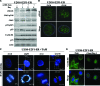
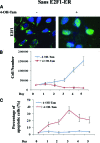
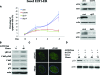
Osteosarcoma is the most common primary bone cancer. Mutations of the RB gene represent the most frequent molecular defect in this malignancy. A major consequence of this alteration is that the activity of the key cell cycle regulator E2F1 is unleashed from the inhibitory effects of pRb. Studies in animal models and in human cancers have shown that deregulated E2F1 overexpression possesses either "oncogenic" or "oncosuppressor" properties, depending on the cellular context. To address this issue in osteosarcomas, we examined the status of E2F1 relative to cell proliferation and apoptosis in a clinical setting of human primary osteosarcomas and in E2F1-inducible osteosarcoma cell line models that are wild-type and deficient for p53. Collectively, our data demonstrated that high E2F1 levels exerted a growth-suppressing effect that relied on the integrity of the DNA damage response network. Surprisingly, induction of p73, an established E2F1 target, was also DNA damage response-dependent. Furthermore, a global proteome analysis associated with bioinformatics revealed novel E2F1-regulated genes and potential E2F1-driven signaling networks that could provide useful targets in challenging this aggressive neoplasm by innovative therapies.
Figures


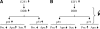
References
-
- Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–989. - PubMed
-
- Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y, Yamamuro T. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994;54:3042–3048. - PubMed
-
- Tsantoulis PK, Gorgoulis VG. Involvement of E2F transcription factor family in cancer. Eur J Cancer. 2005;41:2403–2414. - PubMed
-
- Bell LA, Ryan KM. Life and death decisions by E2F-1. Cell Death Differ. 2004;11:137–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

