Functional amyloids as natural storage of peptide hormones in pituitary secretory granules
- PMID: 19541956
- PMCID: PMC2865899
- DOI: 10.1126/science.1173155
Functional amyloids as natural storage of peptide hormones in pituitary secretory granules
Abstract
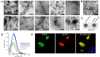
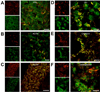
Amyloids are highly organized cross-beta-sheet-rich protein or peptide aggregates that are associated with pathological conditions including Alzheimer's disease and type II diabetes. However, amyloids may also have a normal biological function, as demonstrated by fungal prions, which are involved in prion replication, and the amyloid protein Pmel17, which is involved in mammalian skin pigmentation. We found that peptide and protein hormones in secretory granules of the endocrine system are stored in an amyloid-like cross-beta-sheet-rich conformation. Thus, functional amyloids in the pituitary and other organs can contribute to normal cell and tissue physiology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

