Merkel cells are essential for light-touch responses
- PMID: 19541997
- PMCID: PMC2743005
- DOI: 10.1126/science.1172890
Merkel cells are essential for light-touch responses
Abstract
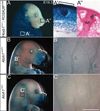
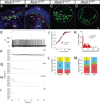
The peripheral nervous system detects different somatosensory stimuli, including pain, temperature, and touch. Merkel cell-neurite complexes are touch receptors composed of sensory afferents and Merkel cells. The role that Merkel cells play in light-touch responses has been the center of controversy for over 100 years. We used Cre-loxP technology to conditionally delete the transcription factor Atoh1 from the body skin and foot pads of mice. Merkel cells are absent from these areas in Atoh1(CKO) animals. Ex vivo skin/nerve preparations from Atoh1(CKO) animals demonstrate complete loss of the characteristic neurophysiologic responses normally mediated by Merkel cell-neurite complexes. Merkel cells are, therefore, required for the proper encoding of Merkel receptor responses, suggesting that these cells form an indispensible part of the somatosensory system.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

