Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation
- PMID: 19542021
- PMCID: PMC2775080
- DOI: 10.1161/ATVBAHA.109.188813
Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation
Abstract
Objective: We investigated whether NADPH oxidase-dependent production of superoxide contributes to activation of NF-kappaB in endothelial cells by the saturated free fatty acid palmitate.
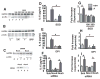
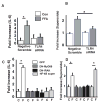
Methods and results: After incubation of human endothelial cells with palmitate at a concentration known to induce cellular inflammation (100 mumol/L), we measured superoxide levels by using electron spin resonance spectroscopy and the spin trap 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH). Palmitate exposure induced a >2-fold increase in superoxide levels, an effect associated with activation of NF-kappaB signaling as measured by phospho-IkappaBalpha, NF-kappaB activity, IL-6, and ICAM expression. Reduction in superoxide levels by each of 3 different interventions-pretreatment with superoxide dismutase (SOD), diphenylene iodinium (DPI), or knockdown of NADPH oxidase 4 (NOX4) by siRNA-attenuated palmitate-mediated NF-kappaB signaling. Inhibition of toll like receptor-4 (TLR4) signaling also suppressed palmitate-mediated superoxide production and associated inflammation, whereas palmitate-mediated superoxide production was not affected by overexpression of a phosphorylation mutant IkappaBalpha (NF-kappaB super repressor) that blocks cellular inflammation downstream of IKKbeta/NF-kappaB. Finally, high-fat feeding increased expression of NOX4 and an upstream activator, bone morphogenic protein (BMP4), in thoracic aortic tissue from C57BL/6 mice, but not in TLR4(-/-) mice, compared to low-fat fed controls.
Conclusions: These results suggest that NADPH oxidase-dependent superoxide production links palmitate-stimulated TLR4 activation to NF-kappaB signaling in endothelial cells.
Figures
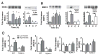

Similar articles
-
Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity.Circ Res. 2007 Jun 8;100(11):1589-96. doi: 10.1161/CIRCRESAHA.106.142851. Epub 2007 May 3. Circ Res. 2007. PMID: 17478729
-
AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes.Circ Res. 2010 Apr 2;106(6):1117-28. doi: 10.1161/CIRCRESAHA.109.212530. Epub 2010 Feb 18. Circ Res. 2010. PMID: 20167927 Free PMC article.
-
Soyasaponin Bb inhibits the recruitment of toll-like receptor 4 (TLR4) into lipid rafts and its signaling pathway by suppressing the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent generation of reactive oxygen species.Mol Nutr Food Res. 2016 Jul;60(7):1532-43. doi: 10.1002/mnfr.201600015. Epub 2016 Apr 21. Mol Nutr Food Res. 2016. PMID: 27005845
-
Palmitate induces interleukin-8 expression in human aortic vascular smooth muscle cells via Toll-like receptor 4/nuclear factor-κB pathway (TLR4/NF-κB-8).J Diabetes. 2014 Jan;6(1):33-41. doi: 10.1111/1753-0407.12073. Epub 2013 Aug 1. J Diabetes. 2014. PMID: 23826669
-
NF- kappa B independent suppression of endothelial vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 gene expression by inhibition of flavin binding proteins and superoxide production.J Mol Cell Cardiol. 2000 Aug;32(8):1499-508. doi: 10.1006/jmcc.2000.1183. J Mol Cell Cardiol. 2000. PMID: 10900176
Cited by
-
NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox.J Biol Chem. 2011 Mar 11;286(10):7779-7787. doi: 10.1074/jbc.M110.200063. Epub 2010 Nov 30. J Biol Chem. 2011. PMID: 21118808 Free PMC article.
-
Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin f(2α) impairs endothelial function in renovascular hypertensive rats.Antioxid Redox Signal. 2012 Feb 15;16(4):363-73. doi: 10.1089/ars.2010.3874. Epub 2011 Dec 2. Antioxid Redox Signal. 2012. PMID: 21951274 Free PMC article.
-
Intravenous Lipid Infusion Induces Endoplasmic Reticulum Stress in Endothelial Cells and Blood Mononuclear Cells of Healthy Adults.J Am Heart Assoc. 2016 Jan 11;5(1):e002574. doi: 10.1161/JAHA.115.002574. J Am Heart Assoc. 2016. PMID: 26755554 Free PMC article.
-
Proinflammatory NFkB signalling promotes mitochondrial dysfunction in skeletal muscle in response to cellular fuel overloading.Cell Mol Life Sci. 2019 Dec;76(24):4887-4904. doi: 10.1007/s00018-019-03148-8. Epub 2019 May 17. Cell Mol Life Sci. 2019. PMID: 31101940 Free PMC article.
-
Ferroptosis in idiopathic pulmonary fibrosis: mechanisms, impact, and therapeutic opportunities.Front Immunol. 2025 May 21;16:1567994. doi: 10.3389/fimmu.2025.1567994. eCollection 2025. Front Immunol. 2025. PMID: 40469306 Free PMC article. Review.
References
-
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–2082. - PubMed
-
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. - PubMed
-
- Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72:447–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

