Loss of positive allosteric interactions between neuronal nitric oxide synthase and phosphofructokinase contributes to defects in glycolysis and increased fatigability in muscular dystrophy
- PMID: 19542095
- PMCID: PMC2729666
- DOI: 10.1093/hmg/ddp288
Loss of positive allosteric interactions between neuronal nitric oxide synthase and phosphofructokinase contributes to defects in glycolysis and increased fatigability in muscular dystrophy
Abstract
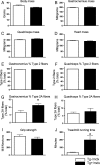
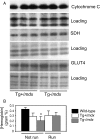
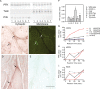
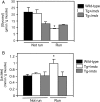
Duchenne muscular dystrophy (DMD) involves a complex pathophysiology that is not easily explained by the loss of the protein dystrophin, the primary defect in DMD. Instead, many features of the pathology are attributable to the secondary loss of neuronal nitric oxide synthase (nNOS) from dystrophin-deficient muscle. In this investigation, we tested whether the loss of nNOS contributes to the increased fatigability of mdx mice, a model of DMD. Our findings show that the expression of a muscle-specific, nNOS transgene increases the endurance of mdx mice and enhances glycogen metabolism during treadmill-running, but did not affect vascular perfusion of muscles. We also find that the specific activity of phosphofructokinase (PFK; the rate limiting enzyme in glycolysis) is positively affected by nNOS in muscle; PFK-specific activity is significantly reduced in mdx muscles and the muscles of nNOS null mutants, but significantly increased in nNOS transgenic muscles and muscles from mdx mice that express the nNOS transgene. PFK activity measured under allosteric conditions was significantly increased by nNOS, but unaffected by endothelial NOS or inducible NOS. The specific domain of nNOS that positively regulates PFK activity was assayed by cloning and expressing different domains of nNOS and assaying their effects on PFK activity. This approach yielded a polypeptide that included the flavin adenine dinucleotide (FAD)-binding domain of nNOS as the region of the molecule that promotes PFK activity. Smaller peptides in this domain were then synthesized and used in activity assays that showed a 36-amino acid peptide in the FAD-binding domain in which most of the positive allosteric activity of nNOS for PFK resides. Mapping this peptide onto the structure of nNOS shows that the peptide is exposed on the surface, readily available for binding. Collectively, these findings indicate that defects in glycolytic metabolism and increased fatigability in dystrophic muscle may be caused in part by the loss of positive allosteric interactions between nNOS and PFK.
Figures
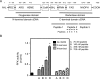
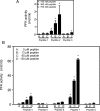

References
-
- Brenman J.E., Chao D.S., Xia H., Aldape K., Bredt D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

