Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants
- PMID: 19542200
- PMCID: PMC2731533
- DOI: 10.2337/db09-0551
Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants
Abstract
Objective: Zinc ions are essential for the formation of hexameric insulin and hormone crystallization. A nonsynonymous single nucleotide polymorphism rs13266634 in the SLC30A8 gene, encoding the secretory granule zinc transporter ZnT8, is associated with type 2 diabetes. We describe the effects of deleting the ZnT8 gene in mice and explore the action of the at-risk allele.
Research design and methods: Slc30a8 null mice were generated and backcrossed at least twice onto a C57BL/6J background. Glucose and insulin tolerance were measured by intraperitoneal injection or euglycemic clamp, respectively. Insulin secretion, electrophysiology, imaging, and the generation of adenoviruses encoding the low- (W325) or elevated- (R325) risk ZnT8 alleles were undertaken using standard protocols.
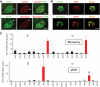
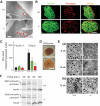
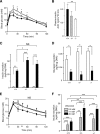
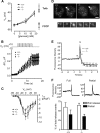
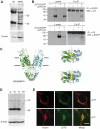
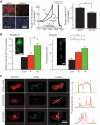
Results: ZnT8(-/-) mice displayed age-, sex-, and diet-dependent abnormalities in glucose tolerance, insulin secretion, and body weight. Islets isolated from null mice had reduced granule zinc content and showed age-dependent changes in granule morphology, with markedly fewer dense cores but more rod-like crystals. Glucose-stimulated insulin secretion, granule fusion, and insulin crystal dissolution, assessed by total internal reflection fluorescence microscopy, were unchanged or enhanced in ZnT8(-/-) islets. Insulin processing was normal. Molecular modeling revealed that residue-325 was located at the interface between ZnT8 monomers. Correspondingly, the R325 variant displayed lower apparent Zn(2+) transport activity than W325 ZnT8 by fluorescence-based assay.
Conclusions: ZnT8 is required for normal insulin crystallization and insulin release in vivo but not, remarkably, in vitro. Defects in the former processes in carriers of the R allele may increase type 2 diabetes risks.
Figures


References
-
- Rutter GA, Parton LE: The β-cell in type 2 diabetes and in obesity. Front Horm Res 2008; 36: 118– 134 - PubMed
-
- Dodson G, Steiner D: The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol 1998; 8: 189– 194 - PubMed
-
- Baker EN, Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DM, Hubbard RE, Isaacs NW, Reynolds CD: The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci 1998; 319: 369– 456 - PubMed
-
- Michael DJ, Ritzel RA, Haataja L, Chow RH: Pancreatic β-cells secrete insulin in fast- and slow-release forms. Diabetes 2006; 55: 600– 607 - PubMed
-
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB: Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 2003; 5: 330– 335 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

