Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice
- PMID: 19542201
- PMCID: PMC2731527
- DOI: 10.2337/db09-0259
Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice
Abstract
Objective: To elucidate the molecular basis for mitochondrial dysfunction, which has been implicated in the pathogenesis of diabetes complications.
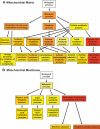
Research design and methods: Mitochondrial matrix and membrane fractions were generated from liver, brain, heart, and kidney of wild-type and type 1 diabetic Akita mice. Comparative proteomics was performed using label-free proteome expression analysis. Mitochondrial state 3 respirations and ATP synthesis were measured, and mitochondrial morphology was evaluated by electron microscopy. Expression of genes that regulate mitochondrial biogenesis, substrate utilization, and oxidative phosphorylation (OXPHOS) were determined.
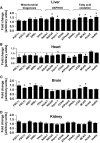
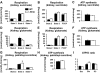
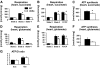
Results: In diabetic mice, fatty acid oxidation (FAO) proteins were less abundant in liver mitochondria, whereas FAO protein content was induced in mitochondria from all other tissues. Kidney mitochondria showed coordinate induction of tricarboxylic acid (TCA) cycle enzymes, whereas TCA cycle proteins were repressed in cardiac mitochondria. Levels of OXPHOS subunits were coordinately increased in liver mitochondria, whereas mitochondria of other tissues were unaffected. Mitochondrial respiration, ATP synthesis, and morphology were unaffected in liver and kidney mitochondria. In contrast, state 3 respirations, ATP synthesis, and mitochondrial cristae density were decreased in cardiac mitochondria and were accompanied by coordinate repression of OXPHOS and peroxisome proliferator-activated receptor (PPAR)-gamma coactivator (PGC)-1alpha transcripts.
Conclusions: Type 1 diabetes causes tissue-specific remodeling of the mitochondrial proteome. Preservation of mitochondrial function in kidney, brain, and liver, versus mitochondrial dysfunction in the heart, supports a central role for mitochondrial dysfunction in diabetic cardiomyopathy.
Figures

References
-
- Borch-Johnsen K: The prognosis of insulin-dependent diabetes mellitus. An epidemiological approach. Dan Med Bull 1989; 36: 336– 348 - PubMed
-
- Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL: The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study: mortality results. Diabetes 1984; 33: 271– 276 - PubMed
-
- Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED: Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes 2008; 57: 2924– 2932 - PMC - PubMed
-
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM: Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001; 413: 131– 138 - PubMed
-
- de Cavanagh EM, Ferder L, Toblli JE, Piotrkowski B, Stella I, Fraga CG, Inserra F: Renal mitochondrial impairment is attenuated by AT1 blockade in experimental type I diabetes. Am J Physiol Heart Circ Physiol 2008; 294: H456– H465 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

