Aqueous accessibility to the transmembrane regions of subunit c of the Escherichia coli F1F0 ATP synthase
- PMID: 19542218
- PMCID: PMC2749098
- DOI: 10.1074/jbc.M109.002501
Aqueous accessibility to the transmembrane regions of subunit c of the Escherichia coli F1F0 ATP synthase
Abstract
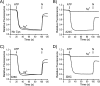
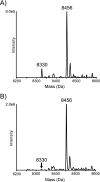
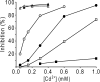
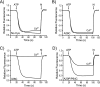
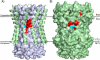
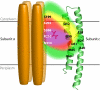
Rotary catalysis in F(1)F(0) ATP synthase is powered by proton translocation through the membrane-embedded F(0) sector. Proton binding and release occur in the middle of the membrane at Asp-61 on transmembrane helix (TMH) 2 of subunit c. Previously the reactivity of Cys substituted into TMH2 revealed extensive aqueous access at the cytoplasmic side as probed with Ag(+) and other thiolate-directed reagents. The analysis of aqueous accessibility of membrane-embedded regions in subunit c was extended here to TMH1 and the periplasmic side of TMH2. The Ag(+) sensitivity of Cys substitutions was more limited on the periplasmic versus cytoplasmic side of TMH2. In TMH1, Ag(+) sensitivity was restricted to a pocket of four residues lying directly behind Asp-61. Aqueous accessibility was also probed using Cd(2+), a membrane-impermeant soft metal ion with properties similar to Ag(+). Cd(2+) inhibition was restricted to the I28C substitution in TMH1 and residues surrounding Asp-61 in TMH2. The overall pattern of inhibition, by all of the reagents tested, indicates highest accessibility on the cytoplasmic side of TMH2 and in a pocket of residues around Asp-61, including proximal residues in TMH1. Additionally subunit a was shown to mediate access to this region by the membrane-impermeant probe 2-(trimethylammonium)ethyl methanethiosulfonate. Based upon these results and other information, a pocket of aqueous accessible residues, bordered by the peripheral surface of TMH4 of subunit a, is proposed to extend from the cytoplasmic side of cTMH2 to Asp-61 in the center of the membrane.
Figures


Similar articles
-
Chemical reactivities of cysteine substitutions in subunit a of ATP synthase define residues gating H+ transport from each side of the membrane.J Biol Chem. 2010 Dec 17;285(51):39811-8. doi: 10.1074/jbc.M110.175844. Epub 2010 Oct 13. J Biol Chem. 2010. PMID: 20943664 Free PMC article.
-
Subunit a facilitates aqueous access to a membrane-embedded region of subunit c in Escherichia coli F1F0 ATP synthase.J Biol Chem. 2008 May 2;283(18):12365-72. doi: 10.1074/jbc.M800901200. Epub 2008 Mar 10. J Biol Chem. 2008. PMID: 18332132 Free PMC article.
-
Aqueous access pathways in ATP synthase subunit a. Reactivity of cysteine substituted into transmembrane helices 1, 3, and 5.J Biol Chem. 2007 Mar 23;282(12):9001-7. doi: 10.1074/jbc.M610848200. Epub 2007 Jan 18. J Biol Chem. 2007. PMID: 17234633
-
Mechanics of coupling proton movements to c-ring rotation in ATP synthase.FEBS Lett. 2003 Nov 27;555(1):29-34. doi: 10.1016/s0014-5793(03)01101-3. FEBS Lett. 2003. PMID: 14630314 Review.
-
Structural model of the transmembrane Fo rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ.Biochim Biophys Acta. 2002 Oct 11;1565(2):232-45. doi: 10.1016/s0005-2736(02)00572-2. Biochim Biophys Acta. 2002. PMID: 12409198 Review.
Cited by
-
A new type of Na(+)-driven ATP synthase membrane rotor with a two-carboxylate ion-coupling motif.PLoS Biol. 2013;11(6):e1001596. doi: 10.1371/journal.pbio.1001596. Epub 2013 Jun 25. PLoS Biol. 2013. PMID: 23824040 Free PMC article.
-
Obstruction of transmembrane helical movements in subunit a blocks proton pumping by F1Fo ATP synthase.J Biol Chem. 2013 Aug 30;288(35):25535-25541. doi: 10.1074/jbc.M113.496794. Epub 2013 Jul 17. J Biol Chem. 2013. PMID: 23864659 Free PMC article.
-
Chemical reactivities of cysteine substitutions in subunit a of ATP synthase define residues gating H+ transport from each side of the membrane.J Biol Chem. 2010 Dec 17;285(51):39811-8. doi: 10.1074/jbc.M110.175844. Epub 2010 Oct 13. J Biol Chem. 2010. PMID: 20943664 Free PMC article.
-
Cryo-EM studies of the structure and dynamics of vacuolar-type ATPases.Sci Adv. 2016 Jul 22;2(7):e1600725. doi: 10.1126/sciadv.1600725. eCollection 2016 Jul. Sci Adv. 2016. PMID: 27532044 Free PMC article. Review.
-
Biophysical Characterization of a Thermoalkaliphilic Molecular Motor with a High Stepping Torque Gives Insight into Evolutionary ATP Synthase Adaptation.J Biol Chem. 2016 Nov 11;291(46):23965-23977. doi: 10.1074/jbc.M116.743633. Epub 2016 Sep 13. J Biol Chem. 2016. PMID: 27624936 Free PMC article.
References
-
- Yoshida M., Muneyuki E., Hisabori T. (2001) Nat. Rev. Mol. Cell Biol. 2,669–677 - PubMed
-
- Capaldi R. A., Aggeler R. (2002) Trends Biochem. Sci. 27,154–160 - PubMed
-
- von Ballmoos C., Cook G. M., Dimroth P. (2008) Annu. Rev. Biophys. 37,43–64 - PubMed
-
- Stock D., Leslie A. G., Walker J. E. (1999) Science 286,1700–1705 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

