Mirk regulates the exit of colon cancer cells from quiescence
- PMID: 19542220
- PMCID: PMC2755699
- DOI: 10.1074/jbc.M109.035519
Mirk regulates the exit of colon cancer cells from quiescence
Abstract
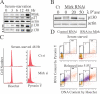
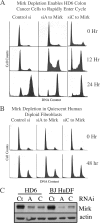
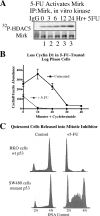
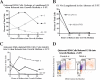
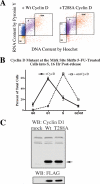
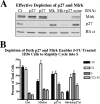
Mirk/Dyrk1B is a serine/threonine kinase widely expressed in colon cancers. Serum starvation induced HD6 colon carcinoma cells to enter a quiescent G0 state, characterized by a 2N DNA content and a lower RNA content than G1 cells. Compared with cycling cells, quiescent cells exhibited 16-fold higher levels of the retinoblastoma protein p130/Rb2, which sequesters E2F4 to block entry into G1, 10-fold elevated levels of the CDK inhibitor p27kip1, and 10-fold higher levels of Mirk. However, depletion of Mirk did not prevent entry into G0, but enabled quiescent HD6, SW480, and colo320 colon carcinoma cells to acquire some biochemical characteristics of G1 cells, including increased levels of cyclin D1 and cyclin D3 because of slower turnover, increased activity of their CDK4/cyclin D complexes, and increased phosphorylation and decreased E2F4 sequestering ability of the CDK4 target, p130/Rb2. As a result, depletion of Mirk allowed some cells to escape quiescence and enabled cells released from quiescence to traverse G1 more quickly. The kinase activity of Mirk was increased by the chemotherapeutic drug 5-fluorouracil (5-FU). Treatment of p53 mutant colon cancer cells with 5-FU led to an elongated G1 in a Mirk-dependent manner, as G1 was shortened by ectopic overexpression of cyclin D1 mutated at the Mirk phosphorylation site (T288A), but not by wild-type cyclin D1. Mirk, through regulating cyclin D turnover, and the CDK inhibitor p27, as shown by depletion studies, functioned independently and additively to regulate the exit of tumor cells from quiescence.
Figures

Similar articles
-
Ovarian cancer cells, not normal cells, are damaged by Mirk/Dyrk1B kinase inhibition.Int J Cancer. 2013 May 15;132(10):2258-69. doi: 10.1002/ijc.27917. Epub 2012 Nov 21. Int J Cancer. 2013. PMID: 23114871 Free PMC article.
-
Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer cells by reducing levels of reactive oxygen species.Cancer Res. 2009 Apr 15;69(8):3317-24. doi: 10.1158/0008-5472.CAN-08-2903. Epub 2009 Apr 7. Cancer Res. 2009. PMID: 19351855 Free PMC article.
-
Transient arrest in a quiescent state allows ovarian cancer cells to survive suboptimal growth conditions and is mediated by both Mirk/dyrk1b and p130/RB2.Int J Cancer. 2011 Jul 15;129(2):307-18. doi: 10.1002/ijc.25692. Epub 2010 Nov 18. Int J Cancer. 2011. PMID: 20857490
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
Mirk/Dyrk1B in cancer.J Cell Biochem. 2007 Oct 1;102(2):274-9. doi: 10.1002/jcb.21451. J Cell Biochem. 2007. PMID: 17583556 Review.
Cited by
-
Targeting DYRK1A/B kinases to modulate p21-cyclin D1-p27 signalling and induce anti-tumour activity in a model of human glioblastoma.J Cell Mol Med. 2021 Nov;25(22):10650-10662. doi: 10.1111/jcmm.17002. Epub 2021 Oct 28. J Cell Mol Med. 2021. PMID: 34708541 Free PMC article.
-
Mimicking Muscle Stem Cell Quiescence in Culture: Methods for Synchronization in Reversible Arrest.Methods Mol Biol. 2017;1556:283-302. doi: 10.1007/978-1-4939-6771-1_15. Methods Mol Biol. 2017. PMID: 28247356
-
The zebrafish dyrk1b gene is important for endoderm formation.Genesis. 2010 Jan;48(1):20-30. doi: 10.1002/dvg.20578. Genesis. 2010. PMID: 20014342 Free PMC article.
-
Depleting Mirk Kinase Increases Cisplatin Toxicity in Ovarian Cancer Cells.Genes Cancer. 2010 Aug 1;1(8):803-811. doi: 10.1177/1947601910377644. Genes Cancer. 2010. PMID: 21113238 Free PMC article.
-
Novel checkpoint pathway organization promotes genome stability in stationary-phase yeast cells.Mol Cell Biol. 2013 Jan;33(2):457-72. doi: 10.1128/MCB.05831-11. Epub 2012 Nov 12. Mol Cell Biol. 2013. PMID: 23149941 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

