Lactogenic hormonal induction of long distance interactions between beta-casein gene regulatory elements
- PMID: 19542223
- PMCID: PMC2755689
- DOI: 10.1074/jbc.M109.032490
Lactogenic hormonal induction of long distance interactions between beta-casein gene regulatory elements
Abstract
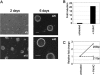
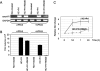
Lactogenic hormone regulation of beta-casein gene expression in mammary epithelial cells provides an excellent model in which to study the mechanisms by which steroid and peptide hormone signaling control gene expression. Prolactin- and glucocorticoid-mediated induction of beta-casein gene expression involves two principal regulatory regions, a proximal promoter and a distal enhancer located in the mouse approximately -6 kb upstream of the transcription start site. Using a chromosome conformation capture assay and quantitative real time PCR, we demonstrate that a chromatin loop is created in conjunction with the recruitment of specific transcription factors and p300 in HC11 mammary epithelial cells. Stimulation with both prolactin and hydrocortisone is required for the induction of these long range interactions between the promoter and enhancer, and no DNA looping was observed in nontreated cells or cells treated with each of the hormones separately. The lactogenic hormone-induced interaction between the proximal promoter and distal enhancer was confirmed in hormone-treated primary three-dimensional mammary acini cultures. In addition, the developmental regulation of DNA looping between the beta-casein regulatory regions was observed in lactating but not in virgin mouse mammary glands. Furthermore, beta-casein mRNA induction and long range interactions between these regulatory regions were inhibited in a progestin-dependent manner following stimulation with prolactin and hydrocortisone in HC11 cells expressing human PR-B. Collectively, these data suggest that the communication between these regulatory regions with intervening DNA looping is a crucial step required to both create and maintain active chromatin domains and regulate transcription.
Figures
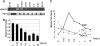



Similar articles
-
Progesterone receptor directly inhibits β-casein gene transcription in mammary epithelial cells through promoting promoter and enhancer repressive chromatin modifications.Mol Endocrinol. 2011 Jun;25(6):955-68. doi: 10.1210/me.2011-0064. Epub 2011 Apr 28. Mol Endocrinol. 2011. PMID: 21527503 Free PMC article.
-
Integration of prolactin and glucocorticoid signaling at the beta-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers.Mol Endocrinol. 2006 Oct;20(10):2355-68. doi: 10.1210/me.2006-0160. Epub 2006 Jun 13. Mol Endocrinol. 2006. PMID: 16772529
-
The nuclear factor YY1 participates in repression of the beta-casein gene promoter in mammary epithelial cells and is counteracted by mammary gland factor during lactogenic hormone induction.Mol Cell Biol. 1994 Jan;14(1):128-37. doi: 10.1128/mcb.14.1.128-137.1994. Mol Cell Biol. 1994. PMID: 8264581 Free PMC article.
-
Hormonal regulation of transcription factor activity in mammary epithelial cells.Mol Cell Endocrinol. 1994 Apr;100(1-2):109-14. doi: 10.1016/0303-7207(94)90288-7. Mol Cell Endocrinol. 1994. PMID: 8056143 Review.
-
Regulatory Domains and Their Mechanisms.Cold Spring Harb Symp Quant Biol. 2015;80:45-51. doi: 10.1101/sqb.2015.80.027268. Epub 2015 Nov 20. Cold Spring Harb Symp Quant Biol. 2015. PMID: 26590168 Review.
Cited by
-
Progesterone receptor directly inhibits β-casein gene transcription in mammary epithelial cells through promoting promoter and enhancer repressive chromatin modifications.Mol Endocrinol. 2011 Jun;25(6):955-68. doi: 10.1210/me.2011-0064. Epub 2011 Apr 28. Mol Endocrinol. 2011. PMID: 21527503 Free PMC article.
-
Cell-specific and shared regulatory elements control a multigene locus active in mammary and salivary glands.Nat Commun. 2023 Aug 17;14(1):4992. doi: 10.1038/s41467-023-40712-0. Nat Commun. 2023. PMID: 37591874 Free PMC article.
-
Transcriptional regulation of FoxO3 gene by glucocorticoids in murine myotubes.Am J Physiol Endocrinol Metab. 2016 Apr 1;310(7):E572-85. doi: 10.1152/ajpendo.00214.2015. Epub 2016 Jan 12. Am J Physiol Endocrinol Metab. 2016. PMID: 26758684 Free PMC article.
-
Regulation of the JAK2-STAT5 Pathway by Signaling Molecules in the Mammary Gland.Front Cell Dev Biol. 2020 Nov 17;8:604896. doi: 10.3389/fcell.2020.604896. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33282878 Free PMC article. Review.
-
Comparison of human coagulation factor VIII expression directed by cytomegalovirus and mammary gland-specific promoters in HC11 cells and transgenic mice.Blood Coagul Fibrinolysis. 2015 Oct;26(7):755-61. doi: 10.1097/MBC.0000000000000318. Blood Coagul Fibrinolysis. 2015. Retraction in: Blood Coagul Fibrinolysis. 2018 Jan;29(1):139. doi: 10.1097/MBC.0000000000000693. PMID: 26192111 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

