Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2
- PMID: 19542234
- PMCID: PMC2785361
- DOI: 10.1074/jbc.M109.026922
Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2
Abstract
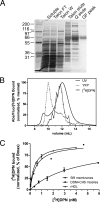
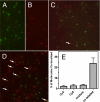
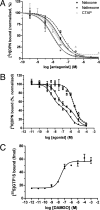
Despite extensive characterization of the mu-opioid receptor (MOR), the biochemical properties of the isolated receptor remain unclear. In light of recent reports, we proposed that the monomeric form of MOR can activate G proteins and be subject to allosteric regulation. A mu-opioid receptor fused to yellow fluorescent protein (YMOR) was constructed and expressed in insect cells. YMOR binds ligands with high affinity, displays agonist-stimulated [(35)S]guanosine 5'-(gamma-thio)triphosphate binding to Galpha(i), and is allosterically regulated by coupled G(i) protein heterotrimer both in insect cell membranes and as purified protein reconstituted into a phospholipid bilayer in the form of high density lipoprotein particles. Single-particle imaging of fluorescently labeled receptor indicates that the reconstituted YMOR is monomeric. Moreover, single-molecule imaging of a Cy3-labeled agonist, [Lys(7), Cys(8)]dermorphin, illustrates a novel method for studying G protein-coupled receptor-ligand binding and suggests that one molecule of agonist binds per monomeric YMOR. Together these data support the notion that oligomerization of the mu-opioid receptor is not required for agonist and antagonist binding and that the monomeric receptor is the minimal functional unit in regard to G protein activation and strong allosteric regulation of agonist binding by G proteins.
Figures
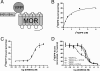

Similar articles
-
Biased μ-opioid receptor agonists diversely regulate lateral mobility and functional coupling of the receptor to its cognate G proteins.Naunyn Schmiedebergs Arch Pharmacol. 2016 Dec;389(12):1289-1300. doi: 10.1007/s00210-016-1293-8. Epub 2016 Sep 6. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 27600870
-
The bovine mu-opioid receptor: cloning of cDNA and pharmacological characterization of the receptor expressed in mammalian cells.Brain Res Mol Brain Res. 1999 Nov 10;73(1-2):129-37. doi: 10.1016/s0169-328x(99)00249-1. Brain Res Mol Brain Res. 1999. PMID: 10581406
-
Mu-delta opioid receptor functional interaction: Insight using receptor-G protein fusions.J Pharmacol Exp Ther. 2006 Aug;318(2):683-90. doi: 10.1124/jpet.106.101220. Epub 2006 May 11. J Pharmacol Exp Ther. 2006. PMID: 16690720
-
Coupling efficacy and selectivity of the human mu-opioid receptor expressed as receptor-Galpha fusion proteins in Escherichia coli.J Neurochem. 2000 Sep;75(3):1190-9. doi: 10.1046/j.1471-4159.2000.0751190.x. J Neurochem. 2000. PMID: 10936202
-
[Effects of newly isolated opioid peptides on G-protein activation: usefulness of [35S] GTP gamma S binding study and its practical application].Nihon Shinkei Seishin Yakurigaku Zasshi. 1998 Aug;18(4):107-16. Nihon Shinkei Seishin Yakurigaku Zasshi. 1998. PMID: 9866825 Review. Japanese.
Cited by
-
Opportunities for functional selectivity in GPCR antibodies.Biochem Pharmacol. 2013 Jan 15;85(2):147-52. doi: 10.1016/j.bcp.2012.08.021. Epub 2012 Sep 10. Biochem Pharmacol. 2013. PMID: 22975405 Free PMC article. Review.
-
Reconstitution of G protein-coupled receptors into a model bilayer system: reconstituted high-density lipoprotein particles.Methods Mol Biol. 2011;756:167-82. doi: 10.1007/978-1-61779-160-4_8. Methods Mol Biol. 2011. PMID: 21870225 Free PMC article.
-
Escaping the flatlands: new approaches for studying the dynamic assembly and activation of GPCR signaling complexes.Trends Pharmacol Sci. 2011 Jul;32(7):410-9. doi: 10.1016/j.tips.2011.03.004. Epub 2011 Apr 15. Trends Pharmacol Sci. 2011. PMID: 21497404 Free PMC article. Review.
-
Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance.Pharmacol Rev. 2013 Jan 15;65(1):223-54. doi: 10.1124/pr.112.005942. Print 2013 Jan. Pharmacol Rev. 2013. PMID: 23321159 Free PMC article. Review.
-
Functional Stability of the Human Kappa Opioid Receptor Reconstituted in Nanodiscs Revealed by a Time-Resolved Scintillation Proximity Assay.PLoS One. 2016 Apr 1;11(4):e0150658. doi: 10.1371/journal.pone.0150658. eCollection 2016. PLoS One. 2016. PMID: 27035823 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

