Characteristics of children intubated and mechanically ventilated in 16 PICUs
- PMID: 19542258
- PMCID: PMC2775993
- DOI: 10.1378/chest.09-0207
Characteristics of children intubated and mechanically ventilated in 16 PICUs
Abstract
Background: When designing multicenter clinical trials, it is important to understand the characteristics of children who have received ventilation in PICUs.
Methods: This study involved the secondary analysis of an existing data set of all children intubated and mechanically ventilated from 16 US PICUs who were initially screened for a multicenter clinical trial on pediatric acute lung injury (ALI).
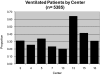
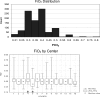
Results: A total of 12,213 children between 2 weeks and 18 years of age who were intubated and mechanically ventilated were included, representing 30% of PICU admissions (center range, 20 to 64%). Of the children who received ventilation, 22% had cyanotic congenital heart disease; 26% had respiratory failure but not bilateral pulmonary infiltrates on chest radiograph; 8% had chronic respiratory disease; 7% had upper airway obstruction; and 5% had reactive airway disease. At least 1,457 patients (15%) with respiratory failure lacked an arterial line. Of these patients, 97% had a positive end-expiratory pressure <or= 8 cm H(2)O, and 80% were supported on an Fio(2) of <or= 0.40. Moreover, 104 of 904 patients (12%) with pulse oximetric saturation (Spo(2)) and Fio(2) measurements available would have met the oxygenation criteria for ALI according to Spo(2)/Fio(2) ratio criteria.
Conclusions: At least 30% of children in a cross-section of US PICUs are endotracheally intubated, and 25% of those with respiratory failure do not fulfill the radiographic criteria for ALI. Although few patients without an indwelling arterial line require more than modest ventilator support, many may still meet the oxygenation criteria for ALI. These findings will facilitate sample size calculations and help to determine feasibility for future trials on pediatric mechanical ventilation.
Figures

References
-
- Randolph AG, Meert KL, O'Neil ME, et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334–1340. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination: the consensus committee. Intensive Care Med. 1994;20:225–232. - PubMed
-
- Khemani RG, Markovitz B, Curley MAQ. Selection of positive end expiratory pressure (PEEP) and fraction of inspired oxygen for mechanically ventilated children without an arterial line [abstract] Pediatr Crit Care Med. 2007;35(suppl):A232.
-
- Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

