Cell envelope perturbation induces oxidative stress and changes in iron homeostasis in Vibrio cholerae
- PMID: 19542276
- PMCID: PMC2725621
- DOI: 10.1128/JB.00092-09
Cell envelope perturbation induces oxidative stress and changes in iron homeostasis in Vibrio cholerae
Abstract
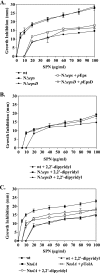
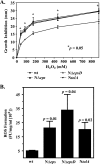
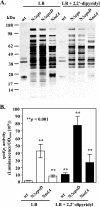
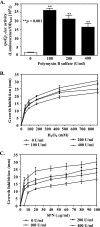
The Vibrio cholerae type II secretion (T2S) machinery is a multiprotein complex that spans the cell envelope. When the T2S system is inactivated, cholera toxin and other exoproteins accumulate in the periplasmic compartment. Additionally, loss of secretion via the T2S system leads to a reduced growth rate, compromised outer membrane integrity, and induction of the extracytoplasmic stress factor RpoE (A. E. Sikora, S. R. Lybarger, and M. Sandkvist, J. Bacteriol. 189:8484-8495, 2007). In this study, gene expression profiling reveals that inactivation of the T2S system alters the expression of genes encoding cell envelope components and proteins involved in central metabolism, chemotaxis, motility, oxidative stress, and iron storage and acquisition. Consistent with the gene expression data, molecular and biochemical analyses indicate that the T2S mutants suffer from internal oxidative stress and increased levels of intracellular ferrous iron. By using a tolA mutant of V. cholerae that shares a similar compromised membrane phenotype but maintains a functional T2S machinery, we show that the formation of radical oxygen species, induction of oxidative stress, and changes in iron physiology are likely general responses to cell envelope damage and are not unique to T2S mutants. Finally, we demonstrate that disruption of the V. cholerae cell envelope by chemical treatment with polymyxin B similarly results in induction of the RpoE-mediated stress response, increased sensitivity to oxidants, and a change in iron metabolism. We propose that many types of extracytoplasmic stresses, caused either by genetic alterations of outer membrane constituents or by chemical or physical damage to the cell envelope, induce common signaling pathways that ultimately lead to internal oxidative stress and misregulation of iron homeostasis.
Figures


Similar articles
-
Compromised outer membrane integrity in Vibrio cholerae Type II secretion mutants.J Bacteriol. 2007 Dec;189(23):8484-95. doi: 10.1128/JB.00583-07. Epub 2007 Sep 21. J Bacteriol. 2007. PMID: 17890307 Free PMC article.
-
A Vibrio cholerae BolA-Like Protein Is Required for Proper Cell Shape and Cell Envelope Integrity.mBio. 2019 Jul 9;10(4):e00790-19. doi: 10.1128/mBio.00790-19. mBio. 2019. PMID: 31289173 Free PMC article.
-
The Type II secretion system delivers matrix proteins for biofilm formation by Vibrio cholerae.J Bacteriol. 2014 Dec;196(24):4245-52. doi: 10.1128/JB.01944-14. Epub 2014 Sep 29. J Bacteriol. 2014. PMID: 25266381 Free PMC article.
-
Alternative sigma factor RpoE is important for Vibrio parahaemolyticus cell envelope stress response and intestinal colonization.Infect Immun. 2014 Sep;82(9):3667-77. doi: 10.1128/IAI.01854-14. Epub 2014 Jun 16. Infect Immun. 2014. PMID: 24935982 Free PMC article.
-
Epidemiology & molecular biology of Vibrio cholerae O139 Bengal.Indian J Med Res. 1996 Jul;104:14-27. Indian J Med Res. 1996. PMID: 8783504 Review.
Cited by
-
Transcriptional profiling of Vibrio cholerae O1 following exposure to human anti- lipopolysaccharide monoclonal antibodies.Pathog Dis. 2020 Jun 1;78(4):ftaa029. doi: 10.1093/femspd/ftaa029. Pathog Dis. 2020. PMID: 32589220 Free PMC article.
-
The Neisseria gonorrhoeae type IV pilus promotes resistance to hydrogen peroxide- and LL-37-mediated killing by modulating the availability of intracellular, labile iron.PLoS Pathog. 2022 Jun 17;18(6):e1010561. doi: 10.1371/journal.ppat.1010561. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35714158 Free PMC article.
-
Jizanpeptins, Cyanobacterial Protease Inhibitors from a Symploca sp. Cyanobacterium Collected in the Red Sea.J Nat Prod. 2018 Jun 22;81(6):1417-1425. doi: 10.1021/acs.jnatprod.8b00117. Epub 2018 May 29. J Nat Prod. 2018. PMID: 29808677 Free PMC article.
-
Proteins secreted via the type II secretion system: smart strategies of Vibrio cholerae to maintain fitness in different ecological niches.PLoS Pathog. 2013 Feb;9(2):e1003126. doi: 10.1371/journal.ppat.1003126. Epub 2013 Feb 21. PLoS Pathog. 2013. PMID: 23436993 Free PMC article. Review. No abstract available.
-
Molecular Mechanisms of Enhanced Bacterial Growth on Hexadecane with Red Clay.Microb Ecol. 2015 Nov;70(4):912-21. doi: 10.1007/s00248-015-0624-5. Epub 2015 May 10. Microb Ecol. 2015. PMID: 25956940
References
-
- Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27215-237. - PubMed
-
- Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50219-230. - PubMed
-
- Berry, M. C., G. C. McGhee, Y. Zhao, and G. W. Sundin. 2009. Effect of a waaL mutation on lipopolysaccharide composition, oxidative stress survival, and virulence in Erwinia amylovora. FEMS Microbiol. Lett. 29180-87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

