Enhancement of the synthesis of RpoE and StpA by polyamines at the level of translation in escherichia coli under heat shock conditions
- PMID: 19542278
- PMCID: PMC2725623
- DOI: 10.1128/JB.00387-09
Enhancement of the synthesis of RpoE and StpA by polyamines at the level of translation in escherichia coli under heat shock conditions
Abstract
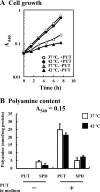
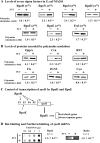
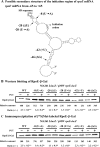
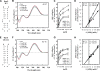
Proteins whose synthesis is enhanced by polyamines at the level of translation were identified with a polyamine-requiring mutant cultured in the presence of 0.1% glucose and 0.02% glutamate at 42 degrees C. Polyamines had a greater effect on cell growth at 42 degrees C than at 37 degrees C. At 42 degrees C, the synthesis of RpoE (sigma(24)) and StpA, which are involved in the transcription of a number of heat shock response genes, was stimulated by polyamines at the level of translation. In the rpoE and stpA mRNAs, a Shine-Dalgarno (SD) sequence is located at 13 and 12 nucleotides, respectively, upstream of the initiation codon AUG. When the SD sequences were moved to the more common position 7 nucleotides upstream of the initiation codon AUG, the degree of polyamine stimulation was reduced, although the level of RpoE and StpA synthesis was markedly increased. The mechanism underlying polyamine stimulation of RpoE synthesis was then studied. Polyamine stimulation of RpoE synthesis was reduced by changing the bulged-out structure in the initiation site of rpoE mRNA, although the level of RpoE synthesis increased. A selective structural change of this bulged-out region induced by spermidine at 42 degrees C was observed by circular dichroism. Polyamine stimulation of fMet-tRNA binding to ribosomes at 42 degrees C also disappeared by changing the bulged-out structure in the initiation site of rpoE mRNA. The results suggest that polyamines enhance the synthesis of RpoE by changing the bulged-out structure in the initiation site of rpoE mRNA.
Figures



Similar articles
-
Effect of Spermidine Analogues on Cell Growth of Escherichia coli Polyamine Requiring Mutant MA261.PLoS One. 2016 Jul 19;11(7):e0159494. doi: 10.1371/journal.pone.0159494. eCollection 2016. PLoS One. 2016. PMID: 27434546 Free PMC article.
-
Cytotoxic Mechanism of Excess Polyamines Functions through Translational Repression of Specific Proteins Encoded by Polyamine Modulon.Int J Mol Sci. 2020 Mar 31;21(7):2406. doi: 10.3390/ijms21072406. Int J Mol Sci. 2020. PMID: 32244348 Free PMC article.
-
Enhancement of the synthesis of RpoN, Cra, and H-NS by polyamines at the level of translation in Escherichia coli cultured with glucose and glutamate.J Bacteriol. 2007 Mar;189(6):2359-68. doi: 10.1128/JB.01562-06. Epub 2007 Jan 12. J Bacteriol. 2007. PMID: 17220219 Free PMC article.
-
Effects of polyamines on protein synthesis and growth of Escherichia coli.J Biol Chem. 2018 Nov 30;293(48):18702-18709. doi: 10.1074/jbc.TM118.003465. Epub 2018 Aug 14. J Biol Chem. 2018. PMID: 30108177 Free PMC article. Review.
-
Modulation of protein synthesis by polyamines.IUBMB Life. 2015 Mar;67(3):160-9. doi: 10.1002/iub.1363. Epub 2015 Apr 23. IUBMB Life. 2015. PMID: 25906835 Review.
Cited by
-
Effects of norspermidine and spermidine on biofilm formation by potentially pathogenic Escherichia coli and Salmonella enterica wild-type strains.Appl Environ Microbiol. 2015 Mar;81(6):2226-32. doi: 10.1128/AEM.03518-14. Epub 2015 Jan 16. Appl Environ Microbiol. 2015. PMID: 25595767 Free PMC article.
-
Effect of Spermidine Analogues on Cell Growth of Escherichia coli Polyamine Requiring Mutant MA261.PLoS One. 2016 Jul 19;11(7):e0159494. doi: 10.1371/journal.pone.0159494. eCollection 2016. PLoS One. 2016. PMID: 27434546 Free PMC article.
-
Ribosome modulation factor, an important protein for cell viability encoded by the polyamine modulon.J Biol Chem. 2010 Sep 10;285(37):28698-707. doi: 10.1074/jbc.M110.111195. Epub 2010 Jul 13. J Biol Chem. 2010. PMID: 20628056 Free PMC article.
-
Cytotoxic Mechanism of Excess Polyamines Functions through Translational Repression of Specific Proteins Encoded by Polyamine Modulon.Int J Mol Sci. 2020 Mar 31;21(7):2406. doi: 10.3390/ijms21072406. Int J Mol Sci. 2020. PMID: 32244348 Free PMC article.
-
Transcriptional profiles of Burkholderia pseudomallei reveal the direct and indirect roles of Sigma E under oxidative stress conditions.BMC Genomics. 2014 Sep 12;15(1):787. doi: 10.1186/1471-2164-15-787. BMC Genomics. 2014. PMID: 25214426 Free PMC article.
References
-
- Adler, J., and B. Templeton. 1967. The effect of environmental conditions on the motility of Escherichia coli. J. Gen. Microbiol. 46175-184. - PubMed
-
- Atkins, J. F., J. B. Lewis, C. W. Anderson, and R. F. Gesteland. 1975. Enhanced differential synthesis of proteins in a mammalian cell-free system by addition of polyamines. J. Biol. Chem. 2505688-5695. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. - PubMed
-
- Claverie, N., and P. S. Mamont. 1989. Comparative antitumor properties in rodents of irreversible inhibitors of l-ornithine decarboxylase, used as such or as prodrugs. Cancer Res. 494466-4471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

