Respiration of Escherichia coli can be fully uncoupled via the nonelectrogenic terminal cytochrome bd-II oxidase
- PMID: 19542282
- PMCID: PMC2725625
- DOI: 10.1128/JB.00562-09
Respiration of Escherichia coli can be fully uncoupled via the nonelectrogenic terminal cytochrome bd-II oxidase
Abstract
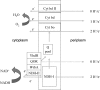
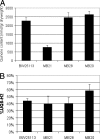
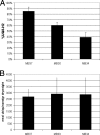
The respiratory chain of Escherichia coli is usually considered a device to conserve energy via the generation of a proton motive force, which subsequently may drive ATP synthesis by the ATP synthetase. It is known that in this system a fixed amount of ATP per oxygen molecule reduced (P/O ratio) is not synthesized due to alternative NADH dehydrogenases and terminal oxidases with different proton pumping stoichiometries. Here we show that P/O ratios can vary much more than previously thought. First, we show that in wild-type E. coli cytochrome bo, cytochrome bd-I, and cytochrome bd-II are the major terminal oxidases; deletion of all of the genes encoding these enzymes results in a fermentative phenotype in the presence of oxygen. Second, we provide evidence that the electron flux through cytochrome bd-II oxidase is significant but does not contribute to the generation of a proton motive force. The kinetics support the view that this system is as an energy-independent system gives the cell metabolic flexibility by uncoupling catabolism from ATP synthesis under non-steady-state conditions. The nonelectrogenic nature of cytochrome bd-II oxidase implies that the respiratory chain can function in a fully uncoupled mode such that ATP synthesis occurs solely by substrate level phosphorylation. As a consequence, the yield with a carbon and energy source can vary five- to sevenfold depending on the electron flux distribution in the respiratory chain. A full understanding and control of this distribution open new avenues for optimization of biotechnological processes.
Figures
Similar articles
-
Cytochrome bd-I in Escherichia coli is less sensitive than cytochromes bd-II or bo'' to inhibition by the carbon monoxide-releasing molecule, CORM-3: N-acetylcysteine reduces CO-RM uptake and inhibition of respiration.Biochim Biophys Acta. 2013 Sep;1834(9):1693-703. doi: 10.1016/j.bbapap.2013.04.019. Epub 2013 Apr 26. Biochim Biophys Acta. 2013. PMID: 23624261 Free PMC article.
-
Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode.Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17320-4. doi: 10.1073/pnas.1108217108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987791 Free PMC article.
-
Oxygen as Acceptor.EcoSal Plus. 2015;6(2):10.1128/ecosalplus.ESP-0012-2015. doi: 10.1128/ecosalplus.ESP-0012-2015. EcoSal Plus. 2015. PMID: 26734697 Free PMC article. Review.
-
Oxoferryl-porphyrin radical catalytic intermediate in cytochrome bd oxidases protects cells from formation of reactive oxygen species.J Biol Chem. 2012 Mar 16;287(12):8830-8. doi: 10.1074/jbc.M111.333542. Epub 2012 Jan 27. J Biol Chem. 2012. PMID: 22287551 Free PMC article.
-
[Cytochrome bd as Antioxidant Redox Enzyme].Mol Biol (Mosk). 2023 Nov-Dec;57(6):1084. Mol Biol (Mosk). 2023. PMID: 38062962 Review. Russian.
Cited by
-
Activators of the glutamate-dependent acid resistance system alleviate deleterious effects of YidC depletion in Escherichia coli.J Bacteriol. 2011 Mar;193(6):1308-16. doi: 10.1128/JB.01209-10. Epub 2011 Jan 7. J Bacteriol. 2011. PMID: 21216990 Free PMC article.
-
Microbial Upgrading of Acetate into Value-Added Products-Examining Microbial Diversity, Bioenergetic Constraints and Metabolic Engineering Approaches.Int J Mol Sci. 2020 Nov 20;21(22):8777. doi: 10.3390/ijms21228777. Int J Mol Sci. 2020. PMID: 33233586 Free PMC article. Review.
-
Cytochrome bd-I in Escherichia coli is less sensitive than cytochromes bd-II or bo'' to inhibition by the carbon monoxide-releasing molecule, CORM-3: N-acetylcysteine reduces CO-RM uptake and inhibition of respiration.Biochim Biophys Acta. 2013 Sep;1834(9):1693-703. doi: 10.1016/j.bbapap.2013.04.019. Epub 2013 Apr 26. Biochim Biophys Acta. 2013. PMID: 23624261 Free PMC article.
-
Uncoupling of substrate-level phosphorylation in Escherichia coli during glucose-limited growth.Appl Environ Microbiol. 2012 Oct;78(19):6908-13. doi: 10.1128/AEM.01507-12. Epub 2012 Jul 27. Appl Environ Microbiol. 2012. PMID: 22843529 Free PMC article.
-
A consolidated analysis of the physiologic and molecular responses induced under acid stress in the legume-symbiont model-soil bacterium Sinorhizobium meliloti.Sci Rep. 2016 Jul 11;6:29278. doi: 10.1038/srep29278. Sci Rep. 2016. PMID: 27404346 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

