igr Genes and Mycobacterium tuberculosis cholesterol metabolism
- PMID: 19542286
- PMCID: PMC2725594
- DOI: 10.1128/JB.00452-09
igr Genes and Mycobacterium tuberculosis cholesterol metabolism
Abstract
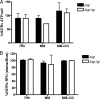
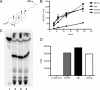
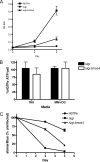
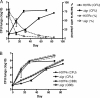
Recently, cholesterol was identified as a physiologically important nutrient for Mycobacterium tuberculosis survival in chronically infected mice. However, it remained unclear precisely when cholesterol is available to the bacterium and what additional bacterial functions are required for its metabolism. Here, we show that the igr locus, which we previously found to be essential for intracellular growth and virulence of M. tuberculosis, is required for cholesterol metabolism. While igr-deficient strains grow identically to the wild type in the presence of short- and long-chain fatty acids, the growth of these bacteria is completely inhibited in the presence of cholesterol. Interestingly, this mutant is still able to respire under cholesterol-dependent growth inhibition, suggesting that the bacteria can metabolize other carbon sources during cholesterol toxicity. Consistent with this hypothesis, we found that the growth-inhibitory effect of cholesterol in vitro depends on cholesterol import, as mutation of the mce4 sterol uptake system partially suppresses this effect. In addition, the Delta igr mutant growth defect during the early phase of disease is completely suppressed by mutating mce4, implicating cholesterol intoxication as the primary mechanism of attenuation. We conclude that M. tuberculosis metabolizes cholesterol throughout infection.
Figures
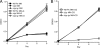
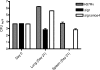
References
-
- Brzostek, A., B. Dziadek, A. Rumijowska-Galewicz, J. Pawelczyk, and J. Dziadek. 2007. Cholesterol oxidase is required for virulence of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 275:106-112. - PubMed
-
- Chang, J. C., N. S. Harik, R. P. Liao, and D. R. Sherman. 2007. Identification of mycobacterial genes that alter growth and pathology in macrophages and in mice. J. Infect. Dis. 196:788-795. - PubMed
-
- Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitheead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. - PubMed
-
- Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

