Genetic analysis of activation of the Vibrio cholerae Cpx pathway
- PMID: 19542291
- PMCID: PMC2725601
- DOI: 10.1128/JB.00406-09
Genetic analysis of activation of the Vibrio cholerae Cpx pathway
Abstract
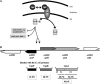
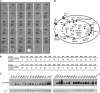
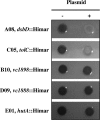
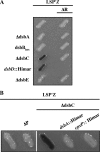
The Cpx two-component system is thought to mediate envelope stress responses in many gram-negative bacteria and has been implicated in the pathogenicity of several enteric pathogens. While cues that activate the Escherichia coli Cpx system have been identified, the nature of the molecular signals that stimulate this pathway is not well understood. Here, we investigated stimuli that trigger this system in Vibrio cholerae, a facultative pathogen that adapts to various niches during its life cycle. In contrast to E. coli, there was no basal activity of the V. cholerae Cpx pathway under standard laboratory conditions. Furthermore, several known stimuli of the E. coli pathway did not induce expression of this system in V. cholerae. There were no defects in intestinal growth in V. cholerae cpx mutants, arguing against the idea that this pathway promotes V. cholerae adaptation to conditions in the mammalian host. We discovered that chloride ions activate the V. cholerae Cpx pathway, raising the possibility that this signal transduction system provides a means for V. cholerae to sense and respond to alterations in salinity. We used a genetic approach to screen for mutants in which the Cpx pathway is activated. We found that mutations in genes whose products are required for periplasmic disulfide bond isomerization result in activation of the Cpx pathway, suggesting that periplasmic accumulation of proteins with aberrant disulfide bonds triggers the V. cholerae Cpx pathway.
Figures



Similar articles
-
Reciprocal regulation of resistance-nodulation-division efflux systems and the Cpx two-component system in Vibrio cholerae.Infect Immun. 2014 Jul;82(7):2980-91. doi: 10.1128/IAI.00025-14. Epub 2014 May 5. Infect Immun. 2014. PMID: 24799626 Free PMC article.
-
The Vibrio cholerae VexGH RND Efflux System Maintains Cellular Homeostasis by Effluxing Vibriobactin.mBio. 2017 May 16;8(3):e00126-17. doi: 10.1128/mBio.00126-17. mBio. 2017. PMID: 28512090 Free PMC article.
-
The Vibrio cholerae Cpx envelope stress response senses and mediates adaptation to low iron.J Bacteriol. 2015 Jan;197(2):262-76. doi: 10.1128/JB.01957-14. Epub 2014 Nov 3. J Bacteriol. 2015. PMID: 25368298 Free PMC article.
-
Just scratching the surface: an expanding view of the Cpx envelope stress response.FEMS Microbiol Lett. 2012 Jan;326(1):2-11. doi: 10.1111/j.1574-6968.2011.02406.x. Epub 2011 Oct 3. FEMS Microbiol Lett. 2012. PMID: 22092948 Review.
-
Vibrio cholerae interactions with the gastrointestinal tract: lessons from animal studies.Curr Top Microbiol Immunol. 2009;337:37-59. doi: 10.1007/978-3-642-01846-6_2. Curr Top Microbiol Immunol. 2009. PMID: 19812979 Review.
Cited by
-
Contribution of the Cpx envelope stress system to metabolism and virulence regulation in Salmonella enterica serovar Typhimurium.PLoS One. 2019 Feb 4;14(2):e0211584. doi: 10.1371/journal.pone.0211584. eCollection 2019. PLoS One. 2019. PMID: 30716090 Free PMC article.
-
Evaluation of CpxRA as a Therapeutic Target for Uropathogenic Escherichia coli Infections.Infect Immun. 2018 Feb 20;86(3):e00798-17. doi: 10.1128/IAI.00798-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29311237 Free PMC article.
-
The crystal structure of the periplasmic domain of Vibrio parahaemolyticus CpxA.Protein Sci. 2012 Sep;21(9):1334-43. doi: 10.1002/pro.2120. Protein Sci. 2012. PMID: 22760860 Free PMC article.
-
Molecular insights into Escherichia coli Cpx envelope stress response activation by the sensor lipoprotein NlpE.Mol Microbiol. 2023 May;119(5):586-598. doi: 10.1111/mmi.15054. Epub 2023 Mar 25. Mol Microbiol. 2023. PMID: 36920223 Free PMC article.
-
The Cpx system regulates virulence gene expression in Vibrio cholerae.Infect Immun. 2015 Jun;83(6):2396-408. doi: 10.1128/IAI.03056-14. Epub 2015 Mar 30. Infect Immun. 2015. PMID: 25824837 Free PMC article.
References
-
- Andersen, C. L., A. Matthey-Dupraz, D. Missiakas, and S. Raina. 1997. A new Escherichia coli gene, dsbG, encodes a periplasmic protein involved in disulphide bond formation, required for recycling DsbA/DsbB and DsbC redox proteins. Mol. Microbiol. 26:121-132. - PubMed
-
- Beckett, C. S., J. A. Loughman, K. A. Karberg, G. M. Donato, W. E. Goldman, and R. G. Kranz. 2000. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol. Microbiol. 38:465-481. - PubMed
-
- Bessette, P. H., J. J. Cotto, H. F. Gilbert, and G. Georgiou. 1999. In vivo and in vitro function of the Escherichia coli periplasmic cysteine oxidoreductase DsbG. J. Biol. Chem. 274:7784-7792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

