Functional genomics of adhesion, invasion, and mycelial formation in Schizosaccharomyces pombe
- PMID: 19542312
- PMCID: PMC2725560
- DOI: 10.1128/EC.00078-09
Functional genomics of adhesion, invasion, and mycelial formation in Schizosaccharomyces pombe
Abstract
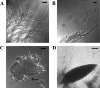
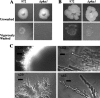
Investigation into the switch between single-celled and filamentous forms of fungi may provide insights into cell polarity, differentiation, and fungal pathogenicity. At the molecular level, much of this investigation has fallen on two closely related budding yeasts, Candida albicans and Saccharomyces cerevisiae. Recently, the much more distant fission yeast Schizosaccharomyces pombe was shown to form invasive filaments after nitrogen limitation (E. Amoah-Buahin, N. Bone, and J. Armstrong, Eukaryot. Cell 4:1287-1297, 2005) and this genetically tractable organism provides an alternative system for the study of dimorphic growth. Here we describe a second mode of mycelial formation of S. pombe, on rich media. Screening of an S. pombe haploid deletion library identified 12 genes required for mycelial development which encode potential transcription factors, orthologues of S. cerevisiae Sec14p and Tlg2p, and the formin For3, among others. These were further grouped into two phenotypic classes representing different stages of the process. We show that galactose-dependent cell adhesion and actin assembly are both required for mycelial formation and mutants lacking a range of genes controlling cell polarity all produce mycelia but with radically altered morphology.
Figures





References
-
- Abeliovich, H., E. Grote, P. Novick, and S. Ferro-Novick. 1998. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J. Biol. Chem. 27311719-11727. - PubMed
-
- Bach, I., C. Carriere, H. P. Ostendorff, B. Andersen, and M. G. Rosenfeld. 1997. A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev. 111370-1380. - PubMed
-
- Chang, F., and M. Peter. 2003. Yeasts make their mark. Nature Cell Biol. 5294-299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

