B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c
- PMID: 19542370
- PMCID: PMC7224988
- DOI: 10.4049/jimmunol.0803706
B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c
Abstract
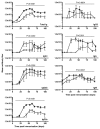
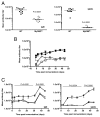
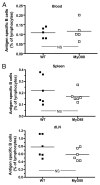
The question of whether Ab responses to T-dependent Ags require B cell intrinsic signaling via the main TLR adaptor (MyD88) has become embroiled in confusion. In part this may be related to the methods used to analyze B cell intrinsic signaling. We have used a mixed bone marrow chimera model to generate mice in which the B cell compartment is completely deficient in MyD88 expression, while the other hematopoietic lineages are largely normal. These mice were immunized with T-dependent Ags or infected with Salmonella. We found that the Ag-specific IgG2c primary response was absolutely dependent on MyD88 signaling to B cells, while other Ig classes were not (IgG1 and IgG3) or much less so (IgG2b, IgA). The MyD88(B-/-) chimeric mice exhibited an impairment of development of IFN-gamma effector T cells, a likely contributory factor in the lack of IgG2c. We also found that B cell intrinsic MyD88 signals are required for the production of natural Abs. The data emphasize the nonredundant role of B cells as programmers of T cell differentiation in vivo.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

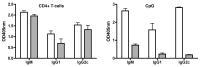

References
-
- Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. - PubMed
-
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. - PubMed
-
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. - PubMed
-
- Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. - PubMed
-
- Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

