Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation
- PMID: 19542372
- PMCID: PMC2754237
- DOI: 10.4049/jimmunol.0900173
Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation
Abstract
The Nlrp3 inflammasome is critical for the activation of caspase-1 in response to danger signals and particulate matter. However, its role in sterile inflammation remains unclear because prestimulation of phagocytic cells with microbial molecules is required for caspase-1 activation. We show here that exposure of macrophages and dendritic cells to TNF-alpha promotes ATP- or silica-mediated caspase-1 activation and IL-1beta secretion in the absence of microbial stimulation. The effect of TNF-alpha was abolished in macrophages deficient in TNF receptor I and II, Nlrp3, or ASC, whereas that induced by TLR ligands required MyD88/Trif. In addition to TNF-alpha, IL-1alpha and IL-1beta promoted caspase-1 activation via Nlrp3 in response to ATP. Remarkably, macrophages tolerized to TNF-alpha, but not to LPS, retained full sensitivity to ATP stimulation via Nlrp3. These results provide a mechanism by which danger signals and particulate matter mediate inflammation via the Nlrp3 inflammasome in the absence of microbial infection.
Conflict of interest statement
Disclosures
The authors declare that no competing interest exist
Figures
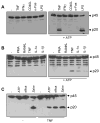

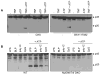
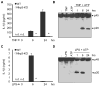
References
-
- Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. - PubMed
-
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. - PubMed
-
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

