Advanced MRI methods for assessment of chronic liver disease
- PMID: 19542391
- PMCID: PMC2989464
- DOI: 10.2214/AJR.09.2601
Advanced MRI methods for assessment of chronic liver disease
Abstract
Objective: With recent advances in technology, advanced MRI methods such as diffusion-weighted and perfusion-weighted MRI, MR elastography, chemical shift-based fat-water separation, and MR spectroscopy can now be applied to liver imaging. We will review the respective roles of these techniques for assessment of chronic liver disease.
Conclusion: MRI plays an increasingly important role in assessment of patients with chronic liver disease because of the lack of ionizing radiation and the possibility of performing multiparametric imaging.
Figures


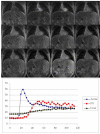

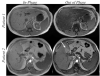

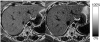



References
-
- Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. - PubMed
-
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. - PubMed
-
- Kim WR, Gross JB, Jr., Poterucha JJ, Locke GR, 3rd, Dickson ER. Outcome of hospital care of liver disease associated with hepatitis C in the United States. Hepatology. 2001;33:201–206. - PubMed
-
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

