The initial phase of an immune response functions to activate regulatory T cells
- PMID: 19542444
- PMCID: PMC2753472
- DOI: 10.4049/jimmunol.0900691
The initial phase of an immune response functions to activate regulatory T cells
Abstract
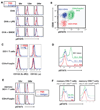
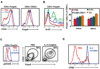
An early reaction of CD4(+) T lymphocytes to Ag is the production of cytokines, notably IL-2. To detect cytokine-dependent responses, naive Ag-specific T cells were stimulated in vivo and the presence of phosphorylated STAT5 molecules was used to identify the cell populations responding to IL-2. Within hours of T cell priming, IL-2-dependent STAT5 phosphorylation occurred primarily in Foxp3(+) regulatory T cells. In contrast, the Ag-specific T cells received STAT5 signals only after repeated Ag exposure or memory differentiation. Regulatory T cells receiving IL-2 signals proliferated and developed enhanced suppressive activity. These results indicate that one of the earliest events in a T cell response is the activation of endogenous regulatory cells, potentially to prevent autoimmunity.
Figures




References
-
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. - PubMed
-
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. - PubMed
-
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

