The transmembrane E3 ligase GRAIL ubiquitinates and degrades CD83 on CD4 T cells
- PMID: 19542455
- PMCID: PMC4300110
- DOI: 10.4049/jimmunol.0900204
The transmembrane E3 ligase GRAIL ubiquitinates and degrades CD83 on CD4 T cells
Abstract
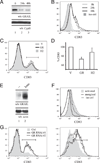
Ubiquitination of eukaryotic proteins regulates a broad range of cellular processes, including T cell activation and tolerance. We have previously demonstrated that GRAIL (gene related to anergy in lymphocytes), a transmembrane RING finger ubiquitin E3 ligase, initially described as induced during the induction of CD4 T cell anergy, is also expressed in resting CD4 T cells. In this study, we show that GRAIL can down-modulate the expression of CD83 (previously described as a cell surface marker for mature dendritic cells) on CD4 T cells. GRAIL-mediated down-modulation of CD83 is dependent on an intact GRAIL extracellular protease-associated domain and an enzymatically active cytosolic RING domain, and proceeds via the ubiquitin-dependent 26S proteosome pathway. Ubiquitin modification of lysine residues K168 and K183, but not K192, in the cytoplasmic domain of CD83 was shown to be necessary for GRAIL-mediated degradation of CD83. Reduced CD83 surface expression levels were seen both on anergized CD4 T cells and following GRAIL expression by retroviral transduction, whereas GRAIL knock-down by RNA interference in CD4 T cells resulted in elevated CD83 levels. Furthermore, CD83 expression on CD4 T cells contributes to T cell activation as a costimulatory molecule. This study supports the novel mechanism of ubiquitination by GRAIL, identifies CD83 as a substrate of GRAIL, and ascribes a role for CD83 in CD4 T cell activation.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





Similar articles
-
GRAIL: a unique mediator of CD4 T-lymphocyte unresponsiveness.FEBS J. 2011 Jan;278(1):47-58. doi: 10.1111/j.1742-4658.2010.07922.x. Epub 2010 Nov 16. FEBS J. 2011. PMID: 21078124 Free PMC article. Review.
-
Herpes simplex virus type 1 ICP0 induces CD83 degradation in mature dendritic cells independent of its E3 ubiquitin ligase function.J Gen Virol. 2014 Jun;95(Pt 6):1366-1375. doi: 10.1099/vir.0.062810-0. Epub 2014 Mar 18. J Gen Virol. 2014. PMID: 24643878
-
The single subunit transmembrane E3 ligase gene related to anergy in lymphocytes (GRAIL) captures and then ubiquitinates transmembrane proteins across the cell membrane.J Biol Chem. 2008 Oct 17;283(42):28497-505. doi: 10.1074/jbc.M805092200. Epub 2008 Aug 18. J Biol Chem. 2008. PMID: 18713730 Free PMC article.
-
CD83 expression is a sensitive marker of activation required for B cell and CD4+ T cell longevity in vivo.J Immunol. 2007 Oct 1;179(7):4550-62. doi: 10.4049/jimmunol.179.7.4550. J Immunol. 2007. PMID: 17878352
-
Role of CD83 in the immunomodulation of dendritic cells.Int Arch Allergy Immunol. 2002 Oct;129(2):113-8. doi: 10.1159/000065883. Int Arch Allergy Immunol. 2002. PMID: 12403928 Review.
Cited by
-
Targeting CD83 for the treatment of graft-versus-host disease.Exp Ther Med. 2013 Jun;5(6):1545-1550. doi: 10.3892/etm.2013.1033. Epub 2013 Apr 2. Exp Ther Med. 2013. PMID: 23837028 Free PMC article.
-
Key biomarkers and latent pathways of dysferlinopathy: Bioinformatics analysis and in vivo validation.Front Neurol. 2022 Sep 20;13:998251. doi: 10.3389/fneur.2022.998251. eCollection 2022. Front Neurol. 2022. PMID: 36203997 Free PMC article.
-
Beyond the Cell Surface: Targeting Intracellular Negative Regulators to Enhance T cell Anti-Tumor Activity.Int J Mol Sci. 2019 Nov 20;20(23):5821. doi: 10.3390/ijms20235821. Int J Mol Sci. 2019. PMID: 31756921 Free PMC article. Review.
-
GRAIL: a unique mediator of CD4 T-lymphocyte unresponsiveness.FEBS J. 2011 Jan;278(1):47-58. doi: 10.1111/j.1742-4658.2010.07922.x. Epub 2010 Nov 16. FEBS J. 2011. PMID: 21078124 Free PMC article. Review.
-
Longitudinal single cell profiling of epitope specific memory CD4+ T cell responses to recombinant zoster vaccine.Nat Commun. 2025 Mar 8;16(1):2332. doi: 10.1038/s41467-025-57562-7. Nat Commun. 2025. PMID: 40057520 Free PMC article.
References
-
- Pickart CM. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. - PubMed
-
- Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. - PubMed
-
- Seroogy CM, Soares L, Ranheim EA, Su L, Holness C, Bloom D, Fathman CG. The gene related to anergy in lymphocytes, an E3 ubiquitin ligase, is necessary for anergy induction in CD4 T cells. J. Immunol. 2004;173:79–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

