Aberrant tissue localization of fungus-specific CD4+ T cells in IL-10-deficient mice
- PMID: 19542472
- PMCID: PMC2753290
- DOI: 10.4049/jimmunol.0900396
Aberrant tissue localization of fungus-specific CD4+ T cells in IL-10-deficient mice
Abstract
Aspergillus fumigatus, a common environmental fungus, can cause lethal invasive infections in immunocompromised hosts. In immunocompetent individuals, however, inhaled A. fumigatus spores prime CD4(+) T cells and activate immune responses that prevent invasive infection. Calibration of inflammatory responses to levels that prevent fungal invasion without inducing collateral tissue damage is essential for host survival, but the underlying regulatory mechanisms remain undefined. Although IL-10 is a validated regulatory cytokine that suppresses immune responses, and IL-10 deficiency or blockade generally enhances immune responses, we find that A. fumigatus-specific T cell frequencies are markedly reduced in airways of IL-10-deficient mice. T cell priming, proliferation, and survival were unaffected by IL-10 deficiency and did not account for decreased frequencies of A. fumigatus-specific T cells in the airways of IL-10-deficient mice. Instead, IL-10 deficiency results in redistribution of A. fumigatus-specific T cells from infected lungs to the gut, a process that is reversed by antibiotic-mediated depletion of intestinal microbes. Our studies demonstrate that disregulated immune responses in the gut can result in dramatic redistribution of pathogen-specific T cells within the host.
Conflict of interest statement
Figures
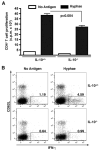




 ) over the whole mouse (Total [T]), in the thoracic region (Trx), or over the abdominal cavity (Abd). Bar graphs are cumulative for signal intensities measured in a total of 15 mice per group in three separate experiments. F, Total number of Af3.16 TCR-tg CD4+ T cells recovered from the airways (BALF) of IL-10−/− (■) and control (
) over the whole mouse (Total [T]), in the thoracic region (Trx), or over the abdominal cavity (Abd). Bar graphs are cumulative for signal intensities measured in a total of 15 mice per group in three separate experiments. F, Total number of Af3.16 TCR-tg CD4+ T cells recovered from the airways (BALF) of IL-10−/− (■) and control ( ) mice and are cumulative of three separate experiments.
) mice and are cumulative of three separate experiments.
Similar articles
-
Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia.Infect Immun. 2005 Nov;73(11):7170-9. doi: 10.1128/IAI.73.11.7170-7179.2005. Infect Immun. 2005. PMID: 16239511 Free PMC article.
-
Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus.J Immunol. 2002 Feb 1;168(3):1362-71. doi: 10.4049/jimmunol.168.3.1362. J Immunol. 2002. PMID: 11801677
-
Aspergillus fumigatus Influences Gasdermin-D-Dependent Pyroptosis of the Lung via Regulating Toll-Like Receptor 2-Mediated Regulatory T Cell Differentiation.J Immunol Res. 2021 Jun 14;2021:5538612. doi: 10.1155/2021/5538612. eCollection 2021. J Immunol Res. 2021. PMID: 34222495 Free PMC article.
-
Immune responses to Aspergillus fumigatus infections.Biol Blood Marrow Transplant. 2006 Jan;12(1 Suppl 1):47-9. doi: 10.1016/j.bbmt.2005.09.007. Biol Blood Marrow Transplant. 2006. PMID: 16399584 Review.
-
Th17 cells in the setting of Aspergillus infection and pathology.Med Mycol. 2009;47 Suppl 1:S162-9. doi: 10.1080/13693780802140766. Epub 2008 Jun 4. Med Mycol. 2009. PMID: 18608926 Review.
Cited by
-
Rodent Models of Invasive Aspergillosis due to Aspergillus fumigatus: Still a Long Path toward Standardization.Front Microbiol. 2017 May 16;8:841. doi: 10.3389/fmicb.2017.00841. eCollection 2017. Front Microbiol. 2017. PMID: 28559881 Free PMC article. Review.
-
Repeated exposure to Aspergillus fumigatus conidia results in CD4+ T cell-dependent and -independent pulmonary arterial remodeling in a mixed Th1/Th2/Th17 microenvironment that requires interleukin-4 (IL-4) and IL-10.Infect Immun. 2012 Jan;80(1):388-97. doi: 10.1128/IAI.05530-11. Epub 2011 Nov 7. Infect Immun. 2012. PMID: 22064716 Free PMC article.
-
Vaccinated C57BL/6 mice develop protective and memory T cell responses to Coccidioides posadasii infection in the absence of interleukin-10.Infect Immun. 2014 Feb;82(2):903-13. doi: 10.1128/IAI.01148-13. Epub 2013 Dec 9. Infect Immun. 2014. PMID: 24478103 Free PMC article.
References
-
- Einsele H, Loeffler J. Contribution of new diagnostic approaches to antifungal treatment plans in high-risk haematology patients. Clin Microbiol Infect. 2008;4 14:37–45. - PubMed
-
- Brakhage AA, Langfelder K. Menacing mold: the molecular biology of Aspergillus fumigatus. Annu Rev Microbiol. 2002;56:433–455. - PubMed
-
- Bozza S, Perruccio K, Montagnoli C, Gaziano R, Bellocchio S, Burchielli E, Nkwanyuo G, Pitzurra L, Velardi A, Romani L. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102:3807–3814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

