Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion
- PMID: 19542476
- PMCID: PMC2742745
- DOI: 10.1164/rccm.200904-0536OC
Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion
Abstract
Rationale: Cavitary disease and delayed culture conversion have been associated with relapse. Combining patient characteristics and measures of bacteriologic response might allow treatment shortening with current drugs in some patients.
Objectives: To assess whether treatment could be shortened from 6 to 4 months in patients with noncavitary tuberculosis whose sputum cultures converted to negative after 2 months.
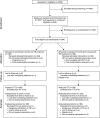
Methods: This study was a randomized, open-label equivalence trial. HIV-uninfected adults with noncavitary tuberculosis were treated daily with isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months, followed by 2 months of isoniazid and rifampin. After 4 months, patients with drug-susceptible TB whose sputum cultures on solid media were negative after 8 weeks of treatment were randomly assigned to continue treatment for 2 more months or to stop treatment. Patients were followed for relapse for 30 months after beginning treatment.
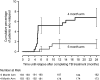
Measurements and main results: Enrollment was stopped by the safety monitoring committee after 394 patients were enrolled due to apparent increased risk for relapse in the 4-month arm. A total of 370 patients were eligible for per protocol analysis. Thirteen patients in the 4-month arm relapsed, compared with three subjects in the 6-month arm (7.0 vs. 1.6%; risk difference, 0.054; 95% confidence interval with Hauck-Anderson correction, 0.01-0.10).
Conclusion: Shortening treatment from 6 to 4 months in adults with noncavitary disease and culture conversion after 2 months using current drugs resulted in a greater relapse rate. The combination of noncavitary disease and 2-month culture conversion was insufficient to identify patients with decreased risk for relapse.
Figures
References
-
- Dutt A, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short course chemotherapy. Am Rev Respir Dis 1989;139:867–870. - PubMed
-
- Hong Kong Chest Service/Tuberculosis Research Centre Madras/British Medical Research Council. A controlled trial of 2-month, 3-month, and 12-month regimens of chemotherapy for sputum smear-negative pulmonary tuberculosis: the results at 30 months. Am Rev Respir Dis 1981;124:138–142. - PubMed
-
- Teo SK, Tan KK, Khoo TK. Four-month chemotherapy in the treatment of smear-negative pulmonary tuberculosis: results at 30 to 60 months. Ann Acad Med Singapore 2002;31:175–181. - PubMed
-
- Hong Kong Chest Service/Tuberculosis Research Centre Madras/British Medical Research Council. A controlled trial of 3-month, 4-month, and 6-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis: results at 5 years. Am Rev Respir Dis 1989;139:871–876. - PubMed
-
- Balasubramanian R, Sivasubramanian S, Vijayan VK, Ramachandran R, Jawahar MS, Paramasivan CN, Selvakumar N, Somasundaram PR. Five year results of a 3-month and two 5-month regimens for the treatment of sputum-positive pulmonary tuberculosis in south India. Tubercle 1990;71:253–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

