Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability
- PMID: 19542489
- PMCID: PMC2724207
- DOI: 10.1152/ajpheart.00318.2009
Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability
Abstract
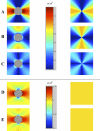
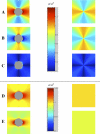
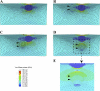
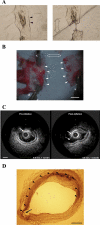
The role of atherosclerotic calcification in plaque rupture remains controversial. In previous analyses using finite element model analysis, circumferential stress was reduced by the inclusion of a calcium deposit in a representative human anatomical configuration. However, a recent report, also using finite element analysis, suggests that microscopic calcium deposits increase plaque stress. We used mathematical models to predict the effects of rigid and liquid inclusions (modeling a calcium deposit and a lipid necrotic core, respectively) in a distensible material (artery wall) on mechanical failure under uniaxial and biaxial loading in a range of configurations. Without inclusions, stress levels were low and uniform. In the analytical model, peak stresses were elevated at the edges of a rigid inclusion. In the finite element model, peak stresses were elevated at the edges of both inclusions, with minimal sensitivity to the wall distensibility and the size and shape of the inclusion. Presence of both a rigid and a soft inclusion enlarged the region of increased wall stress compared with either alone. In some configurations, the rigid inclusion reduced peak stress at the edge of the soft inclusion but simultaneously increased peak stress at the edge of the rigid inclusion and increased the size of the region affected. These findings suggest that the presence of a calcium deposit creates local increases in failure stress, and, depending on relative position to any neighboring lipid pools, it may increase peak stress and the plaque area at risk of mechanical failure.
Figures

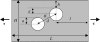
References
-
- Arad Y, Spadaro LA, Roth M, Scordo J, Goodman K, Sherman S, Lledo A, Lerner G, Guerci AD. Correlations between vascular calcification and atherosclerosis: a comparative electron beam CT study of the coronary and carotid arteries. J Comput Assist Tomogr 22: 207–211, 1998. - PubMed
-
- Band W, Goedhard WJ, Knoop AA. Comparison of effects of high cholesterol intake on viscoelastic properties of the thoracic aorta in rats and rabbits. Atherosclerosis 18: 163–171, 1973. - PubMed
-
- Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol 21: 1618–1622, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

