Ziprasidone vs olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial
- PMID: 19542525
- PMCID: PMC3044623
- DOI: 10.1093/schbul/sbp037
Ziprasidone vs olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial
Abstract
Introduction: Head-to-head comparisons of antipsychotics have predominantly included patients with chronic conditions. The aim of the present study was to compare the efficacy and tolerability of ziprasidone and olanzapine in patients with recent-onset schizophrenia.
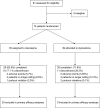
Methods: The study was an 8-week, double-blind, parallel-group, randomized, controlled multicenter trial (NCT00145444). Seventy-six patients with schizophreniform disorder, schizophrenia or schizoaffective disorder (diagnosis < 5 y), and a maximum lifetime antipsychotic treatment < 16 weeks participated in the study. Efficacy of ziprasidone (80-160 mg/d) and olanzapine 10-20 mg was measured using the Positive and Negative Syndrome Scale (PANSS), the Clinical Global Impression (CGI) Scale, the Calgary Depression Scale for Schizophrenia (CDSS), and the Heinrich Quality of Life Scale (HQLS); tolerability assessments included laboratory assessments, body weight, and electroencephalogram.
Results: Olanzapine (n = 34) and ziprasidone (n = 39) showed equal efficacy as measured by the PANSS, CDSS, CGI, and HQLS. However, mean weight gain was significantly higher in the olanzapine group (6.8 vs 0.1 kg, P < .001). Ziprasidone was associated with decreasing levels of triglycerides, cholesterol, and transaminases, while these parameters increased in the olanzapine group (all P values < .05). There were no significant differences in fasting glucose and prolactin levels or in cardiac or sexual side effects. Patients on ziprasidone used biperiden for extrapyramidal side effects more frequently (P < .05).
Discussion: The results of this study indicate that ziprasidone and olanzapine have comparable therapeutic efficacy but differ in their side effect profile. However, there is a risk of a type II error with this sample size. Clinically significant weight gain and laboratory abnormalities appear early after initiating treatment and are more prominent with olanzapine, while more patients on ziprasidone received anticholinergic drugs to treat extrapyramidal symptoms.
Figures

References
-
- Leucht S, Barnes TRE, Kissling W, Engel RR, Correll C, Kane JM. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry. 2003;160:1209–1222. - PubMed
-
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. - PubMed
-
- Robinson DG, Woerner MG, Delman HM, Kane JM. Pharmacological treatments for first-episode schizophrenia. Schizophr Bull. 2005;31:705–722. - PubMed
-
- Duggan L, Fenton M, Dardennes RM, El-Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database Syst Rev. 2005:CD001359. - PubMed

