GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4
- PMID: 19542565
- PMCID: PMC2781314
- DOI: 10.1194/jlr.M900145-JLR200
GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4
Abstract
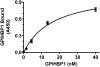
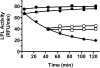
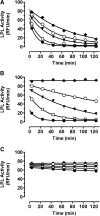
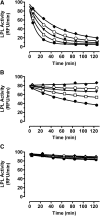
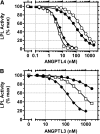
Glycosylphosphatidylinositol-anchored HDL-binding protein (GPIHBP1) binds both LPL and chylomicrons, suggesting that GPIHBP1 is a platform for LPL-dependent processing of triglyceride (TG)-rich lipoproteins. Here, we investigated whether GPIHBP1 affects LPL activity in the absence and presence of LPL inhibitors angiopoietin-like (ANGPTL)3 and ANGPTL4. Like heparin, GPIHBP1 stabilized but did not activate LPL. ANGPTL4 potently inhibited nonstabilized LPL as well as heparin-stabilized LPL but not GPIHBP1-stabilized LPL. Like ANGPTL4, ANGPTL3 inhibited nonstabilized LPL but not GPIHBP1-stabilized LPL. ANGPTL3 also inhibited heparin-stabilized LPL but with less potency than nonstabilized LPL. Consistent with these in vitro findings, fasting serum TGs of Angptl4(-/-)/Gpihbp1(-/-) mice were lower than those of Gpihbp1(-/-) mice and approached those of wild-type littermates. In contrast, serum TGs of Angptl3(-/-)/Gpihbp1(-/-) mice were only slightly lower than those of Gpihbp1(-/-) mice. Treating Gpihbp1(-/-) mice with ANGPTL4- or ANGPTL3-neutralizing antibodies recapitulated the double knockout phenotypes. These data suggest that GPIHBP1 functions as an LPL stabilizer. Moreover, therapeutic agents that prevent LPL inhibition by ANGPTL4 or, to a lesser extent, ANGPTL3, may benefit individuals with hyperlipidemia caused by gene mutations associated with decreased LPL stability.
Figures



Comment in
-
Stabilizing lipoprotein lipase.J Lipid Res. 2009 Dec;50(12):2335-6. doi: 10.1194/jlr.E000703. Epub 2009 Aug 6. J Lipid Res. 2009. PMID: 19661257 Free PMC article. No abstract available.
Similar articles
-
Angiopoietin-like 4 Modifies the Interactions between Lipoprotein Lipase and Its Endothelial Cell Transporter GPIHBP1.J Biol Chem. 2015 May 8;290(19):11865-77. doi: 10.1074/jbc.M114.623769. Epub 2015 Mar 25. J Biol Chem. 2015. PMID: 25809481 Free PMC article.
-
Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL).J Biol Chem. 2009 May 15;284(20):13735-13745. doi: 10.1074/jbc.M807899200. Epub 2009 Mar 23. J Biol Chem. 2009. PMID: 19318355 Free PMC article.
-
Associations of Circulating ANGPTL3, C-Terminal Domain-Containing ANGPTL4, and ANGPTL3/8 and ANGPTL4/8 Complexes with LPL Activity, Diabetes, Inflammation, and Cardiovascular Mortality.Circulation. 2025 Jan 21;151(3):218-234. doi: 10.1161/CIRCULATIONAHA.124.069272. Epub 2024 Oct 11. Circulation. 2025. PMID: 39392008
-
Modulation of plasma TG lipolysis by Angiopoietin-like proteins and GPIHBP1.Biochim Biophys Acta. 2010 Apr;1801(4):415-20. doi: 10.1016/j.bbalip.2009.12.015. Epub 2010 Jan 6. Biochim Biophys Acta. 2010. PMID: 20056168 Review.
-
Regulation of triglyceride metabolism by Angiopoietin-like proteins.Biochim Biophys Acta. 2012 May;1821(5):782-9. doi: 10.1016/j.bbalip.2011.10.010. Epub 2011 Oct 25. Biochim Biophys Acta. 2012. PMID: 22063269 Review.
Cited by
-
Angiopoietin-like 4 Modifies the Interactions between Lipoprotein Lipase and Its Endothelial Cell Transporter GPIHBP1.J Biol Chem. 2015 May 8;290(19):11865-77. doi: 10.1074/jbc.M114.623769. Epub 2015 Mar 25. J Biol Chem. 2015. PMID: 25809481 Free PMC article.
-
Genetic determinants of plasma triglycerides.J Lipid Res. 2011 Feb;52(2):189-206. doi: 10.1194/jlr.R009720. Epub 2010 Nov 1. J Lipid Res. 2011. PMID: 21041806 Free PMC article. Review.
-
Analysis of circulating angiopoietin-like protein 3 and genetic variants in lipid metabolism and liver health: the DiOGenes study.Genes Nutr. 2018 Apr 2;13:7. doi: 10.1186/s12263-018-0597-3. eCollection 2018. Genes Nutr. 2018. PMID: 29619113 Free PMC article.
-
The relationship between plasma angiopoietin-like protein 4 levels, angiopoietin-like protein 4 genotype, and coronary heart disease risk.Arterioscler Thromb Vasc Biol. 2010 Nov;30(11):2277-82. doi: 10.1161/ATVBAHA.110.212209. Epub 2010 Sep 9. Arterioscler Thromb Vasc Biol. 2010. PMID: 20829508 Free PMC article.
-
The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking.Open Biol. 2016 Apr;6(4):150272. doi: 10.1098/rsob.150272. Open Biol. 2016. PMID: 27053679 Free PMC article. Review.
References
-
- Wang J., Ban M. R., Zou G. Y., Cao H., Lin T., Kennedy B. A., Anand S., Yusuf S., Huff M. W., Pollex R. L., et al. 2008. Polygenic determinants of severe hypertriglyceridemia. Hum. Mol. Genet. 17: 2894–2899 - PubMed
-
- Lusis A. J., Pajukanta P. 2008. A treasure trove for lipoprotein biology. Nat. Genet. 40: 129–130 - PubMed
-
- Mead J. R., Irvine S. A., Ramji D. P. 2002. Lipoprotein lipase: structure, function, regulation, and role in disease. J. Mol. Med. 80: 753–769 - PubMed
-
- Merkel M., Eckel R. H., Goldberg I. J. 2002. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43: 1997–2006 - PubMed
-
- Li C.2006. Genetics and regulation of angiopoietin-like proteins 3 and 4. Curr. Opin. Lipidol. 17: 152–156 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

