Drosophila, a golden bug, for the dissection of the genetic basis of tolerance and susceptibility to hypoxia
- PMID: 19542900
- PMCID: PMC6620046
- DOI: 10.1203/PDR.0b013e3181b27275
Drosophila, a golden bug, for the dissection of the genetic basis of tolerance and susceptibility to hypoxia
Abstract
We have previously discovered that the adult Drosophila melanogaster is tolerant to a low O2 environment, withstanding hours of total O2 deprivation without showing any evidence of cell injury. Subsequently, our laboratory embarked on the study of hypoxia tolerance using a mutagenesis and overexpression screens to begin to investigate loss-of-function or gain-of-function phenotypes. Both have given us promising results and, in this article, we detail some of the interesting results. Furthermore, several years ago, we have also started an experimental "Darwinian" selection to generate a fly strain that can perpetuate through all of its life cycle stages in hypoxic environments. Through microarrays and bioinformatic analyses, we have obtained genes (e.g. Notch pathway genes) that play an important role in hypoxia resistance. In addition, we also detail a proof of principle that Drosophila genes that are beneficial in fly resistance to hypoxia can also be as well in mammalian cells. We believe that the mechanisms that we are uncovering in Drosophila will allow us to gain insight regarding susceptibility and tolerance to low O2 and will therefore pave the way to develop better therapies for ailments that afflict humans as a consequence of low O2 delivery or low blood O2 levels.
Figures

 : 4% O2). F)
dMRP4 mRNA expression in pupae. Semi-quantitative RT-PCR was used to determine the expression levels of dMRP4 mRNA from 24B>EP(3)3177 pupae exposed to 4% O2 for 10 days (line 2) or reared at 21% O2 (line 3), yw flies were used as control (line 1). (Adapted with permission from Ma E, et al., J Clin Invest 107:685–693, copyright © 2001, by The American Society for Clinical Investigation, and Huang H and Haddad GG, Physiol Genomics 29:260–266, copyright © 2007 by the American Physiological Society).
: 4% O2). F)
dMRP4 mRNA expression in pupae. Semi-quantitative RT-PCR was used to determine the expression levels of dMRP4 mRNA from 24B>EP(3)3177 pupae exposed to 4% O2 for 10 days (line 2) or reared at 21% O2 (line 3), yw flies were used as control (line 1). (Adapted with permission from Ma E, et al., J Clin Invest 107:685–693, copyright © 2001, by The American Society for Clinical Investigation, and Huang H and Haddad GG, Physiol Genomics 29:260–266, copyright © 2007 by the American Physiological Society).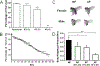
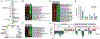

 : AF) (**p<0.01). C) Abolished suppression of genes encoding TCA cycle enzymes in hairy loss-of-function mutants (■: AF; □: h1 mutant;
: AF) (**p<0.01). C) Abolished suppression of genes encoding TCA cycle enzymes in hairy loss-of-function mutants (■: AF; □: h1 mutant;  : h1j3 mutant). D) Significant reduction in hypoxia survival in hairy loss-of-function mutants (open bar: normoxia; gray bar: 6% O2) (**p<0.01). (Adapted with permission from Zhou D et al., PLoS Genet 4:e1000221, copyright © 2008).
: h1j3 mutant). D) Significant reduction in hypoxia survival in hairy loss-of-function mutants (open bar: normoxia; gray bar: 6% O2) (**p<0.01). (Adapted with permission from Zhou D et al., PLoS Genet 4:e1000221, copyright © 2008).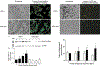
Similar articles
-
Tolerance to low O2: lessons from invertebrate genetic models.Exp Physiol. 2006 Mar;91(2):277-82. doi: 10.1113/expphysiol.2005.030767. Epub 2006 Jan 23. Exp Physiol. 2006. PMID: 16431936
-
Mutation in pre-mRNA adenosine deaminase markedly attenuates neuronal tolerance to O2 deprivation in Drosophila melanogaster.J Clin Invest. 2001 Mar;107(6):685-93. doi: 10.1172/JCI11625. J Clin Invest. 2001. PMID: 11254668 Free PMC article.
-
Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch.PLoS Genet. 2008 Oct;4(10):e1000221. doi: 10.1371/journal.pgen.1000221. Epub 2008 Oct 17. PLoS Genet. 2008. PMID: 18927626 Free PMC article.
-
Understanding the molecular responses to hypoxia using Drosophila as a genetic model.Respir Physiol Neurobiol. 2003 May 30;135(2-3):221-9. doi: 10.1016/s1569-9048(03)00049-1. Respir Physiol Neurobiol. 2003. PMID: 12809621 Review.
-
Genetic analysis of hypoxia tolerance and susceptibility in Drosophila and humans.Annu Rev Genomics Hum Genet. 2013;14:25-43. doi: 10.1146/annurev-genom-091212-153439. Epub 2013 Jun 26. Annu Rev Genomics Hum Genet. 2013. PMID: 23808366 Review.
Cited by
-
Role of Modified Atmosphere in Pest Control and Mechanism of Its Effect on Insects.Front Physiol. 2019 Mar 12;10:206. doi: 10.3389/fphys.2019.00206. eCollection 2019. Front Physiol. 2019. PMID: 30914968 Free PMC article. Review.
-
Matrix metalloproteinase (MMP)-9 induced by Wnt signaling increases the proliferation and migration of embryonic neural stem cells at low O2 levels.J Biol Chem. 2011 May 20;286(20):17649-57. doi: 10.1074/jbc.M111.229427. Epub 2011 Apr 1. J Biol Chem. 2011. PMID: 21460212 Free PMC article.
-
The genetic basis of chronic mountain sickness.Physiology (Bethesda). 2014 Nov;29(6):403-12. doi: 10.1152/physiol.00008.2014. Physiology (Bethesda). 2014. PMID: 25362634 Free PMC article. Review.
-
Aging mechanisms-A perspective mostly from Drosophila.Adv Genet (Hoboken). 2020 Aug 10;1(1):e10026. doi: 10.1002/ggn2.10026. eCollection 2020 Dec. Adv Genet (Hoboken). 2020. PMID: 36619249 Free PMC article.
-
Glial Hsp70 protects K+ homeostasis in the Drosophila brain during repetitive anoxic depolarization.PLoS One. 2011;6(12):e28994. doi: 10.1371/journal.pone.0028994. Epub 2011 Dec 12. PLoS One. 2011. PMID: 22174942 Free PMC article.
References
-
- Haddad GG, Jiang C 1993. Mechanisms of anoxia-induced depolarization in brainstem neurons: in vitro current and voltage clamp studies in the adult rat. Brain Res 625:261–268 - PubMed
-
- Banasiak KJ, Haddad GG 1998. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res 797:295–304 - PubMed
-
- Banasiak KJ, Cronin T, Haddad GG 1999. bcl-2 prolongs neuronal survival during hypoxia-induced apoptosis. Brain Res Mol Brain Res 72:214–225 - PubMed
-
- Banasiak KJ, Xia Y, Haddad GG 2000. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol 62:215–249 - PubMed
-
- Bier E 2005. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet 6:9–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

