Circuits controlling vertebrate locomotion: moving in a new direction
- PMID: 19543221
- PMCID: PMC2847453
- DOI: 10.1038/nrn2608
Circuits controlling vertebrate locomotion: moving in a new direction
Abstract
Neurobiologists have long sought to understand how circuits in the nervous system are organized to generate the precise neural outputs that underlie particular behaviours. The motor circuits in the spinal cord that control locomotion, commonly referred to as central pattern generator networks, provide an experimentally tractable model system for investigating how moderately complex ensembles of neurons generate select motor behaviours. The advent of novel molecular and genetic techniques coupled with recent advances in our knowledge of spinal cord development means that a comprehensive understanding of how the motor circuitry is organized and operates may be within our grasp.
Figures

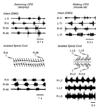

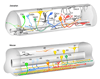

References
-
- Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. How animals move: an integrative view. Science. 2000;288:100–106. - PubMed
-
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interactions. Physiol. Rev. 1975;55:247–304. - PubMed
-
- Orlovsky GN, Deliagina TG, Grillner S. From mollusc to man. New York: Oxford University Press; 1999. Neural Control of Locomotion. This text provides a good introduction and overview of the neural control of locomotion.
-
- Marder E, Bucher D.Central Pattern generators and the control of rhythmic movements Curr. Biol 200111R986–R996.This is an outstanding review that outlines many of the basic principles that operate in rhythmic motor systems. It nicely reviews the invertebrate and vertebrate literature to give an integrative overview of how CPGs are organized and operate. - PubMed
-
- Grillner S.The motor infrastructure: from ion channels to neuronal networks Nat. Rev. Neurosci 20034573–586.This excellent review focuses primarily on the lamprey and outlines the critical findings and principles that underlie the rhythmic motor patterns that control swimming movements. It provides a detailed overview of the swimming CPG and should be read in conjunction with this review. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

