Linear polyubiquitination: a new regulator of NF-kappaB activation
- PMID: 19543231
- PMCID: PMC2727427
- DOI: 10.1038/embor.2009.144
Linear polyubiquitination: a new regulator of NF-kappaB activation
Abstract
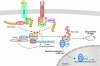
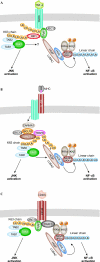
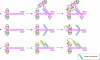
The ubiquitin-conjugation system regulates a vast range of biological phenomena by affecting protein function mostly through polyubiquitin conjugation. The type of polyubiquitin chain that is generated seems to determine how conjugated proteins are regulated, as they are recognized specifically by proteins that contain chain-specific ubiquitin-binding motifs. An enzyme complex that catalyses the formation of newly described linear polyubiquitin chains--known as linear ubiquitin chain-assembly complex (LUBAC)--has recently been characterized, as has a particular ubiquitin-binding domain that specifically recognizes linear chains. Both have been shown to have crucial roles in the canonical nuclear factor-kappaB (NF-kappaB)-activation pathway. The ubiquitin system is intimately involved in regulating the NF-kappaB pathway, and the regulatory roles of K63-linked chains have been studied extensively. However, the role of linear chains in this process is only now emerging. This article discusses the possible mechanisms underlying linear polyubiquitin-mediated activation of NF-kappaB, and the different roles that K63-linked and linear chains have in NF-kappaB activation. Future directions for linear polyubiquitin research are also discussed.
Figures



References
-
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R (2003) Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424: 797–801 - PubMed
-
- Brzovic PS, Rajagopal P, Hoyt DW, King MC, Klevit RE (2001) Structure of a BRCA1–BARD1 heterodimeric RING–RING complex. Nat Struct Biol 8: 833–837 - PubMed
-
- Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A (1989) A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243: 1576–1583 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

