MS80, a novel sulfated oligosaccharide, inhibits pulmonary fibrosis by targeting TGF-beta1 both in vitro and in vivo
- PMID: 19543300
- PMCID: PMC4006651
- DOI: 10.1038/aps.2009.86
MS80, a novel sulfated oligosaccharide, inhibits pulmonary fibrosis by targeting TGF-beta1 both in vitro and in vivo
Abstract
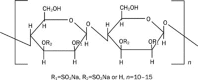
Aim: The pro-fibrogenic cytokine transforming growth factor-beta 1 (TGF-beta1) has attracted much attention for its potential role in the etiology of idiopathic pulmonary fibrosis (IPF). Here, we demonstrate that MS80, a novel sulfated oligosaccharide extracted from seaweed, can bind TGF-beta1. The aim of the present study was to determine whether MS80 is capable of combating TGF-beta1-mediated pulmonary fibrotic events both in vitro and in vivo, and to investigate the possible underlying mechanisms.
Methods: Surface plasmon resonance was used to uncover the binding profiles between the compound and TGF-beta. MTT assay, flow cytometry, Western blot analysis, BCA protein assay and SDS-PAGE gelatin zymography were used to probe the antifibrotic mechanisms of MS80. The in vivo fibrotic efficacy was evaluated in a bleomycin instillation-induced rat model.
Results: We report that MS80, a new kind of sulfated oligosaccharide extracted from seaweed, inhibits TGF-beta1-induced pulmonary fibrosis in vitro and bleomycin-induced pulmonary fibrosis in vivo. Our results indicated that MS80 competitively inhibited heparin/HS-TGF-beta1 interaction through its high binding affinity for TGF-beta1. Moreover, MS80 arrested TGF-beta1-induced human embryo pulmonary fibroblast (HEPF) cell proliferation, collagen deposition and matrix metalloproteinase (MMP) activity. Intriguingly, MS80 deactivated both the ERK and p38 signaling pathways. MS80 was also a potent suppressor of bleomycin-induced rat pulmonary fibrosis in vivo, as evidenced by improved pathological settings and decreased lung collagen contents.
Conclusion: MS80 in particular, and perhaps oligosaccharide in general, offer better pharmacological profiles with appreciably few side effects and represent a promising class of drug candidates for IPF therapy.Acta Pharmacologica Sinica (2009) 30: 973-979; doi: 10.1038/aps.2009.86; published online 22 June 2009.
Figures


Similar articles
-
Glycosyltransferases and glycosaminoglycans in bleomycin and transforming growth factor-β1-induced pulmonary fibrosis.Am J Respir Cell Mol Biol. 2014 Mar;50(3):583-94. doi: 10.1165/rcmb.2012-0226OC. Am J Respir Cell Mol Biol. 2014. PMID: 24127863
-
Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems.Phytomedicine. 2020 Nov;78:153298. doi: 10.1016/j.phymed.2020.153298. Epub 2020 Aug 1. Phytomedicine. 2020. PMID: 32781391 Free PMC article.
-
Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition.Exp Physiol. 2019 Dec;104(12):1942-1951. doi: 10.1113/EP088028. Epub 2019 Oct 23. Exp Physiol. 2019. PMID: 31535412
-
Modifiers of TGF-β1 effector function as novel therapeutic targets of pulmonary fibrosis.Korean J Intern Med. 2014 May;29(3):281-90. doi: 10.3904/kjim.2014.29.3.281. Epub 2014 Apr 29. Korean J Intern Med. 2014. PMID: 24851060 Free PMC article. Review.
-
Proteasomal regulation of pulmonary fibrosis.Proc Am Thorac Soc. 2010 Feb;7(1):77-83. doi: 10.1513/pats.200906-055JS. Proc Am Thorac Soc. 2010. PMID: 20160152 Free PMC article. Review.
Cited by
-
SP-1 regulation of MMP-9 expression requires Ser586 in the PEST domain.Biochem J. 2012 Jul 15;445(2):229-36. doi: 10.1042/BJ20120053. Biochem J. 2012. PMID: 22519702 Free PMC article.
-
ERK1/2 Signaling Pathway Activated by EGF Promotes Proliferation, Transdifferentiation, and Migration of Cultured Primary Newborn Rat Lung Fibroblasts.Biomed Res Int. 2020 Oct 5;2020:7176169. doi: 10.1155/2020/7176169. eCollection 2020. Biomed Res Int. 2020. PMID: 33083482 Free PMC article.
-
Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts.Respir Res. 2011 Nov 29;12(1):154. doi: 10.1186/1465-9921-12-154. Respir Res. 2011. PMID: 22126332 Free PMC article.
-
Association of the proliferation of lung fibroblasts with the ERK1/2 signaling pathway in neonatal rats with hyperoxia-induced lung fibrosis.Exp Ther Med. 2019 Jan;17(1):701-708. doi: 10.3892/etm.2018.6999. Epub 2018 Nov 21. Exp Ther Med. 2019. PMID: 30651853 Free PMC article.
-
Multi-Pharmaceutical Activities of Chinese Herbal Polysaccharides in the Treatment of Pulmonary Fibrosis: Concept and Future Prospects.Front Pharmacol. 2021 Aug 17;12:707491. doi: 10.3389/fphar.2021.707491. eCollection 2021. Front Pharmacol. 2021. PMID: 34489700 Free PMC article. Review.
References
-
- Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–19. - PubMed
-
- Khalil N, O'Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:155–62. - PubMed
-
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

