Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines
- PMID: 19543319
- PMCID: PMC3030987
- DOI: 10.1038/onc.2009.168
Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines
Abstract
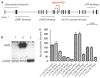
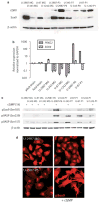
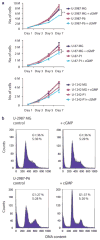
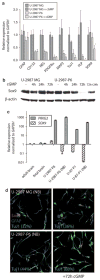
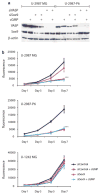
Earlier we used a glioma model to identify loci in the mouse genome, which were repeatedly targeted by platelet-derived growth factor (PDGF)-containing Moloney murine leukemia viruses. The gene Prkg2, encoding cyclic guanosine monophosphate (cGMP)-dependent protein kinase II, cGKII, was tagged by retroviral insertions in two brain tumors. The insertions were both situated upstream of the kinase domain and suggested creating a truncated form of the cGKII protein. We transfected different human glioma cell lines with Prkg2 and found an overall reduction in colony formation and cell proliferation compared with controls transfected with truncated Prkg2 (lacking the kinase domain) or empty vector. All glioma cells transfected with the cGKII phosphorylate vasodilator-stimulated phosphoprotein, VASP, after cGMP analog treatment. Glioma cell lines positive for the Sox9 transcription factor showed reduced Sox9 expression when Prkg2 was stably transfected. When cGKII was activated by cGMP analog treatment, Sox9 was phosphorylated, Sox9 protein expression was suppressed and the glioma cell lines displayed loss of cell adhesion, inhibition of Akt phosphorylation and G1 arrest. Sox9 repression by siRNA was similarly shown to reduce glioma cell proliferation. Expression analysis of stem and glial lineage cell markers also suggests that cGKII induces differentiation of glioma cell lines. These findings describe an anti-proliferative role of cGKII in human glioma biology and would further explain the retroviral tagging of the cGKII gene during brain tumor formation in PDGF-induced tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Phosphorylation of GSK-3beta by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes.J Clin Invest. 2008 Jul;118(7):2506-15. doi: 10.1172/JCI35243. J Clin Invest. 2008. PMID: 18551195 Free PMC article.
-
Cyclic GMP-dependent protein kinase II is a molecular switch from proliferation to hypertrophic differentiation of chondrocytes.Genes Dev. 2004 Oct 1;18(19):2418-29. doi: 10.1101/gad.1224204. Genes Dev. 2004. PMID: 15466490 Free PMC article.
-
Type 2 cGMP-dependent protein kinase regulates proliferation and differentiation in the colonic mucosa.Am J Physiol Gastrointest Liver Physiol. 2012 Jul 15;303(2):G209-19. doi: 10.1152/ajpgi.00500.2011. Epub 2012 May 3. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22556146
-
Function of cGMP-dependent protein kinases in the nervous system.Rev Neurosci. 2005;16(1):23-41. doi: 10.1515/revneuro.2005.16.1.23. Rev Neurosci. 2005. PMID: 15810652 Review.
-
Insights into cGMP signalling derived from cGMP kinase knockout mice.Front Biosci. 2005 May 1;10:1279-89. doi: 10.2741/1618. Front Biosci. 2005. PMID: 15769624 Review.
Cited by
-
The Role of Network Science in Glioblastoma.Cancers (Basel). 2021 Mar 2;13(5):1045. doi: 10.3390/cancers13051045. Cancers (Basel). 2021. PMID: 33801334 Free PMC article. Review.
-
Transcription Factor-Forced Astrocytic Differentiation Impairs Human Glioblastoma Growth In Vitro and In Vivo.Mol Cancer Ther. 2023 Feb 1;22(2):274-286. doi: 10.1158/1535-7163.MCT-21-0903. Mol Cancer Ther. 2023. PMID: 36508391 Free PMC article.
-
Decreased expression of SOX9 indicates a better prognosis and inhibits the growth of glioma cells by inducing cell cycle arrest.Int J Clin Exp Pathol. 2015 Sep 1;8(9):10130-8. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617720 Free PMC article.
-
Restoring soluble guanylyl cyclase expression and function blocks the aggressive course of glioma.Mol Pharmacol. 2011 Dec;80(6):1076-84. doi: 10.1124/mol.111.073585. Epub 2011 Sep 9. Mol Pharmacol. 2011. PMID: 21908708 Free PMC article.
-
Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126.Exp Neurol. 2012 Jun;235(2):427-35. doi: 10.1016/j.expneurol.2011.11.035. Epub 2011 Dec 8. Exp Neurol. 2012. PMID: 22178324 Free PMC article. Review.
References
-
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, et al. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. - PubMed
-
- Cen B, Deguchi A, Weinstein IB. Activation of protein kinase G increases the expression of p21CIP1, p27KIP1, and histidine triad protein 1 through Sp1. Cancer Res. 2008;68:5355–5362. - PubMed
-
- Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

