A tether for Woronin body inheritance is associated with evolutionary variation in organelle positioning
- PMID: 19543374
- PMCID: PMC2690989
- DOI: 10.1371/journal.pgen.1000521
A tether for Woronin body inheritance is associated with evolutionary variation in organelle positioning
Abstract
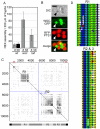
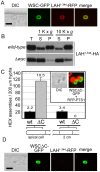
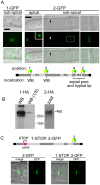
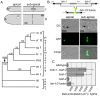
Eukaryotic organelles evolve to support the lifestyle of evolutionarily related organisms. In the fungi, filamentous Ascomycetes possess dense-core organelles called Woronin bodies (WBs). These organelles originate from peroxisomes and perform an adaptive function to seal septal pores in response to cellular wounding. Here, we identify Leashin, an organellar tether required for WB inheritance, and associate it with evolutionary variation in the subcellular pattern of WB distribution. In Neurospora, the leashin (lah) locus encodes two related adjacent genes. N-terminal sequences of LAH-1 bind WBs via the WB-specific membrane protein WSC, and C-terminal sequences are required for WB inheritance by cell cortex association. LAH-2 is localized to the hyphal apex and septal pore rim and plays a role in colonial growth. In most species, WBs are tethered directly to the pore rim, however, Neurospora and relatives have evolved a delocalized pattern of cortex association. Using a new method for the construction of chromosomally encoded fusion proteins, marker fusion tagging (MFT), we show that a LAH-1/LAH-2 fusion can reproduce the ancestral pattern in Neurospora. Our results identify the link between the WB and cell cortex and suggest that splitting of leashin played a key role in the adaptive evolution of organelle localization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Cytoplasmic streaming in neurospora: disperse the plug to increase the flow?PLoS Genet. 2009 Jun;5(6):e1000526. doi: 10.1371/journal.pgen.1000526. Epub 2009 Jun 19. PLoS Genet. 2009. PMID: 19543384 Free PMC article. No abstract available.
Similar articles
-
Making two organelles from one: Woronin body biogenesis by peroxisomal protein sorting.J Cell Biol. 2008 Jan 28;180(2):325-39. doi: 10.1083/jcb.200705049. J Cell Biol. 2008. PMID: 18227279 Free PMC article.
-
Polarized gene expression determines woronin body formation at the leading edge of the fungal colony.Mol Biol Cell. 2005 Jun;16(6):2651-9. doi: 10.1091/mbc.e04-10-0937. Epub 2005 Mar 30. Mol Biol Cell. 2005. PMID: 15800068 Free PMC article.
-
Functional characterization of the Woronin body protein WscA of the pathogenic mold Aspergillus fumigatus.Int J Med Microbiol. 2016 May;306(3):165-73. doi: 10.1016/j.ijmm.2016.03.008. Epub 2016 Mar 18. Int J Med Microbiol. 2016. PMID: 27016805
-
Architecture and development of the Neurospora crassa hypha -- a model cell for polarized growth.Fungal Biol. 2011 Jun;115(6):446-74. doi: 10.1016/j.funbio.2011.02.008. Epub 2011 Feb 19. Fungal Biol. 2011. PMID: 21640311 Review.
-
Recent advances in septum biogenesis in Neurospora crassa.Adv Genet. 2013;83:99-134. doi: 10.1016/B978-0-12-407675-4.00003-1. Adv Genet. 2013. PMID: 23890213 Review.
Cited by
-
Mushrooms: morphological complexity in the fungi.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11655-6. doi: 10.1073/pnas.1006430107. Epub 2010 Jun 22. Proc Natl Acad Sci U S A. 2010. PMID: 20571118 Free PMC article. No abstract available.
-
A large nonconserved region of the tethering protein Leashin is involved in regulating the position, movement, and function of Woronin bodies in Aspergillus oryzae.Eukaryot Cell. 2014 Jul;13(7):866-77. doi: 10.1128/EC.00060-14. Epub 2014 May 9. Eukaryot Cell. 2014. PMID: 24813188 Free PMC article.
-
Woronin bodies move dynamically and bidirectionally by hitchhiking on early endosomes in Aspergillus nidulans.bioRxiv [Preprint]. 2023 Jan 21:2023.01.20.524968. doi: 10.1101/2023.01.20.524968. bioRxiv. 2023. Update in: Mol Biol Cell. 2023 Jun 1;34(7):br9. doi: 10.1091/mbc.E23-01-0025. PMID: 36711994 Free PMC article. Updated. Preprint.
-
The Mycelium as a Network.Microbiol Spectr. 2017 May;5(3):10.1128/microbiolspec.funk-0033-2017. doi: 10.1128/microbiolspec.FUNK-0033-2017. Microbiol Spectr. 2017. PMID: 28524023 Free PMC article. Review.
-
The HEX1 gene of Fusarium graminearum is required for fungal asexual reproduction and pathogenesis and for efficient viral RNA accumulation of Fusarium graminearum virus 1.J Virol. 2013 Sep;87(18):10356-67. doi: 10.1128/JVI.01026-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864619 Free PMC article.
References
-
- Alexopolous CJ, Mims CW, Blackwell M. Introductory Mycology. John Wiley & Sons; 1996.
-
- Lutzoni F, Kauff F, Cox CJ, McLaughlin DJ, Celio G, et al. Assembling the fungal tree of life: Progress, Classification, and evolution of subcellular traits. American Journal of Botany. 2004;9:1446–1480. - PubMed
-
- Muller WH, Montijn RC, Humbel BM, van Aelst AC, Boon EJMC, et al. Structural differences between two types of basidiomycete septal pore caps. Microbiology. 1998;144:1721–1730. - PubMed
-
- Dhavale T, Jedd G. The Fungal Woronin body. In: Howard RJ, Gow NAR, editors. Biology of the fungal cell. Berlin: Springer; 2007. pp. 87–96.
-
- Kirk PM, Cannon PF, David JC, Stalpers JA. Ainsworth and Bisby's Dictionary of the Fungi. Wallingford, UK: CAB International Publishing; 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

