Intravenous inoculation of a bat-associated rabies virus causes lethal encephalopathy in mice through invasion of the brain via neurosecretory hypothalamic fibers
- PMID: 19543379
- PMCID: PMC2691950
- DOI: 10.1371/journal.ppat.1000485
Intravenous inoculation of a bat-associated rabies virus causes lethal encephalopathy in mice through invasion of the brain via neurosecretory hypothalamic fibers
Abstract
The majority of rabies virus (RV) infections are caused by bites or scratches from rabid carnivores or bats. Usually, RV utilizes the retrograde transport within the neuronal network to spread from the infection site to the central nervous system (CNS) where it replicates in neuronal somata and infects other neurons via trans-synaptic spread. We speculate that in addition to the neuronal transport of the virus, hematogenous spread from the site of infection directly to the brain after accidental spill over into the vascular system might represent an alternative way for RV to invade the CNS. So far, it is unknown whether hematogenous spread has any relevance in RV pathogenesis. To determine whether certain RV variants might have the capacity to invade the CNS from the periphery via hematogenous spread, we infected mice either intramuscularly (i.m.) or intravenously (i.v.) with the dog-associated RV DOG4 or the silver-haired bat-associated RV SB. In addition to monitoring the progression of clinical signs of rabies we used immunohistochemistry and quantitative reverse transcription polymerase chain reaction (qRT-PCR) to follow the spread of the virus from the infection site to the brain. In contrast to i.m. infection where both variants caused a lethal encephalopathy, only i.v. infection with SB resulted in the development of a lethal infection. While qRT-PCR did not reveal major differences in virus loads in spinal cord or brain at different times after i.m. or i.v. infection of SB, immunohistochemical analysis showed that only i.v. administered SB directly infected the forebrain. The earliest affected regions were those hypothalamic nuclei, which are connected by neurosecretory fibers to the circumventricular organs neurohypophysis and median eminence. Our data suggest that hematogenous spread of SB can lead to a fatal encephalopathy through direct retrograde invasion of the CNS at the neurovascular interface of the hypothalamus-hypophysis system. This alternative mode of virus spread has implications for the post exposure prophylaxis of rabies, particularly with silver-haired bat-associated RV.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
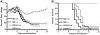


Similar articles
-
Unique characteristics of bat rabies viruses in big brown bats (Eptesicus fuscus).Arch Virol. 2013 Apr;158(4):809-20. doi: 10.1007/s00705-012-1551-0. Epub 2012 Dec 4. Arch Virol. 2013. PMID: 23208279
-
Rabies in a captive colony of big brown bats (Eptesicus fuscus).J Wildl Dis. 2004 Jul;40(3):403-13. doi: 10.7589/0090-3558-40.3.403. J Wildl Dis. 2004. PMID: 15465706
-
Pathogenesis of rabies.Curr Top Microbiol Immunol. 2005;292:45-56. doi: 10.1007/3-540-27485-5_3. Curr Top Microbiol Immunol. 2005. PMID: 15981467 Review.
-
Identification of viral genomic elements responsible for rabies virus neuroinvasiveness.Proc Natl Acad Sci U S A. 2004 Nov 16;101(46):16328-32. doi: 10.1073/pnas.0407289101. Epub 2004 Nov 1. Proc Natl Acad Sci U S A. 2004. PMID: 15520387 Free PMC article.
-
The application of reverse genetics technology in the study of rabies virus (RV) pathogenesis and for the development of novel RV vaccines.J Neurovirol. 2005 Feb;11(1):76-81. doi: 10.1080/13550280590900436. J Neurovirol. 2005. PMID: 15804964 Review.
Cited by
-
Histopathology and immunohistochemistry of tissues outside central nervous system in bovine rabies.J Neurovirol. 2014 Aug;20(4):388-97. doi: 10.1007/s13365-014-0255-5. Epub 2014 Jun 10. J Neurovirol. 2014. PMID: 24912572
-
The spread and evolution of rabies virus: conquering new frontiers.Nat Rev Microbiol. 2018 Apr;16(4):241-255. doi: 10.1038/nrmicro.2018.11. Epub 2018 Feb 26. Nat Rev Microbiol. 2018. PMID: 29479072 Free PMC article. Review.
-
Microglia and a functional type I IFN pathway are required to counter HSV-1-driven brain lateral ventricle enlargement and encephalitis.J Immunol. 2013 Mar 15;190(6):2807-17. doi: 10.4049/jimmunol.1203265. Epub 2013 Feb 4. J Immunol. 2013. PMID: 23382563 Free PMC article.
-
Update on rabies.Res Rep Trop Med. 2011 Feb 2;2:31-43. doi: 10.2147/RRTM.S16013. eCollection 2011. Res Rep Trop Med. 2011. PMID: 30881178 Free PMC article. Review.
-
Establishment of a longitudinal pre-clinical model of lyssavirus infection.J Virol Methods. 2020 Jul;281:113882. doi: 10.1016/j.jviromet.2020.113882. Epub 2020 May 12. J Virol Methods. 2020. PMID: 32407866 Free PMC article.
References
-
- Pringle CR. The order Mononegavirales. Arch Virol. 1991;117:137–140. - PubMed
-
- Bulenga G, Heaney T. Post-exposure local treatment of mice infected with rabies with two axonal flow inhibitors, colchicine and vinblastine. J Gen Virol. 1978;39:381–385. - PubMed
-
- Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999;414:167–192. - PubMed
-
- Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103:63–71. - PubMed
-
- Smith JS, Fishbein DB, Rupprecht CE, Clark K. Unexplained rabies in three immigrants in the United States. A virologic investigation. N Engl J Med. 1991;324:205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

