Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia
- PMID: 19543386
- PMCID: PMC2694354
- DOI: 10.1371/journal.pone.0005978
Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia
Abstract
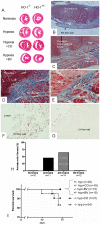
Background: Hypoxia and pressure-overload induce heme oxygenase-1 (HO-1) in cardiomyocytes and vascular smooth muscle cells (VSMCs). HO-1(-/-) mice exposed to chronic hypoxia develop pulmonary arterial hypertension (PAH) with exaggerated right ventricular (RV) injury consisting of dilation, fibrosis, and mural thrombi. Our objective was to identify the HO-1 product(s) mediating RV protection from hypoxic injury in HO-1(-/-) mice.
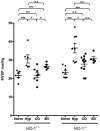
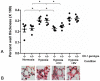
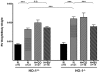
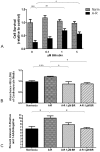
Methodology/principal findings: HO-1(-/-) mice were exposed to seven weeks of hypoxia and treated with inhaled CO or biliverdin injections. CO reduced right ventricular systolic pressure (RVSP) and prevented hypoxic pulmonary arteriolar remodeling in both HO-1(-/-) and control mice. Biliverdin had no significant effect on arteriolar remodeling or RVSP in either genotype. Despite this, biliverdin prevented RV failure in the hypoxic HO-1(-/-) mice (0/14 manifested RV wall fibrosis or thrombus), while CO-treated HO-1(-/-) mice developed RV insults similar to untreated controls. In vitro, CO inhibited hypoxic VSMC proliferation and migration but did not prevent cardiomyocyte death from anoxia-reoxygenation (A-R). In contrast, bilirubin limited A-R-induced cardiomyocyte death but did not inhibit VSMC proliferation and migration.
Conclusions/significance: CO and bilirubin have distinct protective actions in the heart and pulmonary vasculature during chronic hypoxia. Moreover, reducing pulmonary vascular resistance may not prevent RV injury in hypoxia-induced PAH; supporting RV adaptation to hypoxia and preventing RV failure must be a therapeutic goal.
Conflict of interest statement
Figures

References
-
- Duckers HJ, Boehm M, True AL, Yet SF, San H, et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. - PubMed
-
- Zhang M, Zhang BH, Chen L, An W. Overexpression of heme oxygenase-1 protects smooth muscle cells against oxidative injury and inhibits cell proliferation. Cell Res. 2002;12:123–132. - PubMed
-
- Foo RS, Siow RC, Brown MJ, Bennett MR. Heme oxygenase-1 gene transfer inhibits angiotensin II-mediated rat cardiac myocyte apoptosis but not hypertrophy. J Cell Physiol. 2006;209:1–7. - PubMed
-
- Hu CM, Chen YH, Chiang MT, Chau LY. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation. 2004;110:309–316. - PubMed
-
- Tongers J, Fiedler B, Konig D, Kempf T, Klein G, et al. Heme oxygenase-1 inhibition of MAP kinases, calcineurin/NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovasc Res. 2004;63:545–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

