Mineralization potential of polarized dental enamel
- PMID: 19543391
- PMCID: PMC2694366
- DOI: 10.1371/journal.pone.0005986
Mineralization potential of polarized dental enamel
Abstract
Background: Management of human teeth has moved from a surgical to a more conservative approach of inhibiting or preventing lesion progression. Increasing enamel mineralization is crucial in this regard. A potential difficulty is the preferential mineralization of the outermost portion of the enamel that can prevent overall mineralization. We describe a strategy for increasing the mineralization potential of dental enamel.
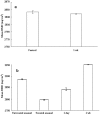
Methodology/principal findings: Extracted human premolar teeth enamel (n = 5) were exposed to a high concentration of hydrogen peroxide with an energizing source. Samples were stored in artificial saliva at 37 degrees C for 1 wk. A desktop X-ray micro-CT system was used to evaluate the mineral density of samples. Mineral distribution was polarized between the lower and the higher mineralized portion of enamel by charged oxygen free radicals due to activation of permeated hydrogen peroxide. The kinetics of energy absorption in the deeper enamel region demonstrated improvement of preferential mineralization into the region without restricting overall mineralization of the enamel. Subsequent increasing mineralization, even in the dense mineralized outer portion of enamel, was also achieved.
Conclusions/significance: This increased mineralization may promote resistance to acidic deterioration of the structure. The present study is one of the primary steps towards the development of novel application in reparative and restorative dentistry.
Conflict of interest statement
Figures


Similar articles
-
Micro-CT analysis of naturally arrested brown spot enamel lesions.J Dent. 2017 Jan;56:105-111. doi: 10.1016/j.jdent.2016.11.007. Epub 2016 Nov 21. J Dent. 2017. PMID: 27884718
-
Quantitative characterization and micro-CT mineral mapping of natural fissural enamel lesions.J Dent. 2016 Mar;46:23-9. doi: 10.1016/j.jdent.2016.01.012. Epub 2016 Feb 2. J Dent. 2016. PMID: 26836704
-
In vitro demineralization of tooth enamel subjected to two whitening regimens.J Am Dent Assoc. 2013 Jul;144(7):799-807. doi: 10.14219/jada.archive.2013.0190. J Am Dent Assoc. 2013. PMID: 23813261
-
[Factors that modify de- and remineralization in dental enamel from the aspect of caries susceptibility].Ann Acad Med Stetin. 1999;Suppl 47:1-89. Ann Acad Med Stetin. 1999. PMID: 10462837 Review. Polish.
-
[Local enamel demineralization diagnostics and treatment].Stomatologiia (Mosk). 2017;96(4):67-71. doi: 10.17116/stomat201796467-71. Stomatologiia (Mosk). 2017. PMID: 28858285 Review. Russian.
References
-
- Zonghan X, Michael S, Paul M, Hoffman M. On the critical parameters that regulate the deformation behavior of tooth enamel. Biomaterials. 2008;29:2697–2703. - PubMed
-
- Huang TT, Jones AS, He LH, Darendeliler MA, Swain MV. Characterisation of enamel white spot lesions using X-ray micro-tomography. J Dent. 2007;35:737–743. - PubMed
-
- Yamaguchi K, Miyazaki M, Takamizawa T, Inage H, Moore B. Effect of CPP-ACP paste on mechanical properties of bovine enamel as determined by an ultrasonic device. J Dent. 2006;34:230–236. - PubMed
-
- Simmelink JW. Ultrastructural effects of diphosphonates on dental enamel. Adv Dent Res. 1987;1:356–365. - PubMed
-
- Weatherell JA, Deutsch D, Robinson C, Hallsworth AS. Assimilation of fluoride by enamel throughout the life of the tooth. Caries Res. 1977;11:85–115. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

