Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function
- PMID: 19543526
- PMCID: PMC2694997
- DOI: 10.1371/journal.pone.0005959
Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function
Abstract
Background: The systemic injection of neural stem/precursor cells (NPCs) provides remarkable amelioration of the clinico-pathological features of experimental autoimmune encephalomyelitis (EAE). This is dependent on the capacity of transplanted NPCs to engage concurrent mechanisms of action within specific microenvironments in vivo. Among a wide range of therapeutic actions alternative to cell replacement, neuroprotective and immune modulatory capacities of transplanted NPCs have been described. However, lacking is a detailed understanding of the mechanisms by which NPCs exert their therapeutic plasticity. This study was designed to identify the first candidate that exemplifies and sustains the immune modulatory capacity of transplanted NPCs.
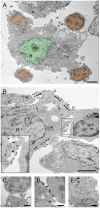
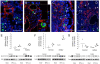
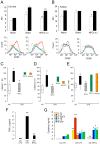
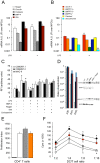
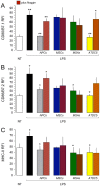
Methodology/principal findings: To achieve the exclusive targeting of the peripheral immune system, SJL mice with PLP-induced EAE were injected subcutaneously with NPCs and the treatment commenced prior to disease onset. NPC-injected EAE mice showed significant clinical improvement, as compared to controls. Exogenous NPCs lacking the expression of major neural antigens were reliably (and for long-term) found at the level of draining lymph nodes, while establishing sophisticated anatomical interactions with lymph node cells. Importantly, injected NPCs were never found in organs other than lymph nodes, including the brain and the spinal cord. Draining lymph nodes from transplanted mice showed focal up-regulation of major developmental stem cell regulators, such as BMP-4, Noggin and Sonic hedgehog. In lymph nodes, injected NPCs hampered the activation of myeloid dendritic cells (DCs) and steadily restrained the expansion of antigen-specific encephalitogenic T cells. Both ex vivo and in vitro experiments identified a novel highly NPC-specific-BMP-4-dependent-mechanism hindering the DC maturation.
Conclusion/significance: The study described herein, identifies the first member of the TGF beta/BMP family of stem cell regulators as a novel tolerogenic factor released by NPCs. Full exploitation of this pathway as an efficient tool for vaccination therapy in autoimmune inflammatory conditions is underway.
Conflict of interest statement
Figures


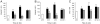
References
-
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. - PubMed
-
- Ben-Hur T, Goldman SA. Prospects of cell therapy for disorders of myelin. Ann N Y Acad Sci. 2008;1142:218–249. - PubMed
-
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. - PubMed
-
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, et al. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–1082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

