Intravital imaging of tumor apoptosis with FRET probes during tumor therapy
- PMID: 19543775
- PMCID: PMC5987252
- DOI: 10.1007/s11307-009-0235-y
Intravital imaging of tumor apoptosis with FRET probes during tumor therapy
Abstract
Purpose: The aim of the study is to dynamically and non-invasively monitor the apoptosis events in vivo during photodynamic therapy (PDT) and chemotherapy.
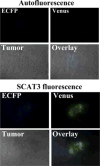
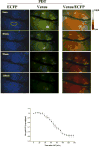
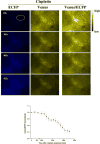
Procedures: A FRET probe, SCAT3, was utilized to determine activation of caspase-3 during tumor cell apoptosis in mice, induced by PDT, and cisplatin treatments. Using this method, dynamics of caspase-3 activation was observed both in vitro and in vivo.
Results: Analysis of the fluorescent missions from tumor cells indicated that the caspase-3 activation started immediately after PDT treatment. In contrast, the caspase-3 activation started about 13 and 36 h after cisplatin treatment in vitro and in vivo, respectively.
Conclusions: FRET could be used effectively to monitor activation of caspase-3 in living organism. This method could be used to provide rapid assessment of apoptosis induced by anti-tumor therapies for improvement of treatment efficacy.
Figures



References
-
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. - PubMed
-
- Blankenberg FG, Katsikis PD, Storrs RW, Beaulieu C, Spielman D, Chen JY, Naumovski L, Tait JF. Quantitative analysis of apoptotic cell death using proton nuclear magnetic resonance spectroscopy. Blood. 1997;89:3778–3786. - PubMed
-
- Aboagye EO, Bhujwalla ZM, Shungu DC, Glickson JD. Detection of tumor response to chemotherapy by 1H nuclear magnetic resonance spectroscopy: effect of 5-fluorouracil on lactate levels in radiation-induced fibrosarcoma 1 tumors. Cancer Res. 1998;58:1063–1067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

