Subacute exposure to N-ethyl perfluorooctanesulfonamidoethanol results in the formation of perfluorooctanesulfonate and alters superoxide dismutase activity in female rats
- PMID: 19544052
- PMCID: PMC2755535
- DOI: 10.1007/s00204-009-0450-y
Subacute exposure to N-ethyl perfluorooctanesulfonamidoethanol results in the formation of perfluorooctanesulfonate and alters superoxide dismutase activity in female rats
Abstract
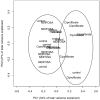
Perfluorooctanesulfonamides, such as N-ethyl perfluorooctanesulfonamidoethanol (N-EtFOSE), are large scale industrial chemicals but their disposition and toxicity are poorly understood despite significant human exposure. The hypothesis that subacute exposure to N-EtFOSE, a weak peroxisome proliferator, causes a redox imbalance in vivo was tested using the known peroxisome proliferator, ciprofibrate, as a positive control. Female Sprague-Dawley rats were treated orally with N-EtFOSE, ciprofibrate or corn oil (vehicle) for 21 days, and levels of N-EtFOSE and its metabolites as well as markers of peroxisome proliferation and oxidative stress were assessed in serum, liver and/or uterus. The N-EtFOSE metabolite profile in liver and serum was in good agreement with reported in vitro biotransformation pathways in rats and the metabolite levels decreasing in the order perfluorooctanesulfonate >> perfluorooctanesulfonamide ~ N-ethyl perfluorooctanesulfonamidoacetate >> perfluorooctanesulfonamidoethanol approximately N-EtFOSE. Although N-EtFOSE treatment significantly decreased the growth rate, increased relative liver weight and activity of superoxide dismutases (SOD) in liver and uterus (total SOD, CuZnSOD and MnSOD), a metabolic study revealed no differences in the metabolome in serum from N-EtFOSE-treated and control animals. Ciprofibrate treatment increased liver weight and peroxisomal acyl Co-A oxidase activity in the liver and altered antioxidant enzyme activities in the uterus and liver. According to NMR metabolomic studies, ciprofibrate treated animals had altered serum lipid profiles compared to N-EtFOSE-treated and control animals, whereas putative markers of peroxisome proliferation in serum were not affected. Overall, this study demonstrates the biotransformation of N-EtFOSE to PFOS in rats that is accompanied by N-EtFOSE-induced alterations in antioxidant enzyme activity.
Conflict of interest statement
Figures




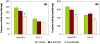
Similar articles
-
Perfluorooctanesulfonate (PFOS) Conversion from N-Ethyl-N-(2-hydroxyethyl)-perfluorooctanesulfonamide (EtFOSE) in male Sprague Dawley rats after inhalation exposure.Environ Res. 2017 May;155:307-313. doi: 10.1016/j.envres.2017.02.029. Epub 2017 Mar 10. Environ Res. 2017. PMID: 28260617
-
Biotransformation of N-ethyl-N-(2-hydroxyethyl)perfluorooctanesulfonamide by rat liver microsomes, cytosol, and slices and by expressed rat and human cytochromes P450.Chem Res Toxicol. 2004 Jun;17(6):767-75. doi: 10.1021/tx034222x. Chem Res Toxicol. 2004. PMID: 15206897
-
Perfluorooctanoate, perflourooctanesulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis.Toxicol Lett. 2002 Mar 24;129(1-2):23-32. doi: 10.1016/s0378-4274(01)00466-0. Toxicol Lett. 2002. PMID: 11879971
-
Accumulation, biodegradation and toxicological effects of N-ethyl perfluorooctane sulfonamidoethanol on the earthworms Eisenia fetida exposed to quartz sands.Ecotoxicol Environ Saf. 2019 Oct 15;181:138-145. doi: 10.1016/j.ecoenv.2019.05.062. Epub 2019 Jun 5. Ecotoxicol Environ Saf. 2019. PMID: 31176248
-
PFOS or PreFOS? Are perfluorooctane sulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctane sulfonate (PFOS) exposure?J Environ Monit. 2010 Nov;12(11):1979-2004. doi: 10.1039/c0em00295j. Epub 2010 Oct 13. J Environ Monit. 2010. PMID: 20944836 Review.
Cited by
-
Model and cell membrane partitioning of perfluorooctanesulfonate is independent of the lipid chain length.Colloids Surf B Biointerfaces. 2010 Mar 1;76(1):128-36. doi: 10.1016/j.colsurfb.2009.10.025. Epub 2009 Oct 27. Colloids Surf B Biointerfaces. 2010. PMID: 19932010 Free PMC article.
-
Risk to human health related to the presence of perfluoroalkyl substances in food.EFSA J. 2020 Sep 17;18(9):e06223. doi: 10.2903/j.efsa.2020.6223. eCollection 2020 Sep. EFSA J. 2020. PMID: 32994824 Free PMC article.
-
Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction.Antioxid Redox Signal. 2015 Jan 1;22(1):78-91. doi: 10.1089/ars.2014.5929. Antioxid Redox Signal. 2015. PMID: 24949841 Free PMC article.
-
Disruption of phosphatidylcholine monolayers and bilayers by perfluorobutane sulfonate.J Phys Chem B. 2012 Aug 23;116(33):9999-10007. doi: 10.1021/jp304412p. Epub 2012 Aug 13. J Phys Chem B. 2012. PMID: 22834732 Free PMC article.
-
Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status.Int J Environ Res Public Health. 2018 Dec 11;15(12):2818. doi: 10.3390/ijerph15122818. Int J Environ Res Public Health. 2018. PMID: 30544905 Free PMC article. Review.
References
-
- Aebi H. Catalase in vitro. In: Packer L, editor. Methods in Enzymology. Academic Press; 1984. pp. 121–126. - PubMed
-
- Ala-Korpela M. Critical evaluation of 1H NMR metabonomics of serum as a methodology for disease risk assessment and diagnostics. Clin Chem Lab Med. 2008;46:27–42. - PubMed
-
- Anderson ME. Tissue glutathione. In: Greenwald R, editor. Methods for oxygen radical research. CRC Press; Boca Raton: 1985. pp. 317–323.
-
- Arrendale RF, Stewart JT, Manning R, Vitayavirasuk B. Determination of GX 071 and its major metabolite in rat blood by cold on-column injection capillary GC/ECD. J Agric Food Chem. 1989;37:1130–1135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

