Total synthesis of cyathin A(3) and cyathin B(2)
- PMID: 19544340
- PMCID: PMC3523203
- DOI: 10.1002/anie.200901669
Total synthesis of cyathin A(3) and cyathin B(2)
Abstract
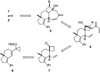
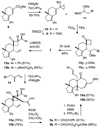
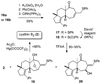
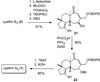
A stereoselective synthesis of cyathin A(3) and cyathin B(2) has been achieved by a Prins-type reaction of a cycloalkenyl cyclopropanol. Particularly noteworthy is the use of a spirocyclobutanone moiety as a convenient scaffold for an efficient ring-closing metathesis to stereoselectively construct a suitably functionalized seven-membered ring (see scheme).
Figures
Similar articles
-
Enantioselective total synthesis of (-)-cyathin B2.J Antibiot (Tokyo). 2014 Jun;67(6):483-5. doi: 10.1038/ja.2014.23. Epub 2014 Apr 2. J Antibiot (Tokyo). 2014. PMID: 24690907 No abstract available.
-
Formal synthesis of (+/-)-guanacastepene A: a tandem ring-closing metathesis approach.Org Lett. 2004 May 27;6(11):1817-20. doi: 10.1021/ol049452x. Org Lett. 2004. PMID: 15151422
-
A general approach to cyathin diterpenes. Total synthesis of allocyathin B(3).Org Lett. 2000 Jul 13;2(14):2125-7. doi: 10.1021/ol006026c. Org Lett. 2000. PMID: 10891246
-
Stemarane Diterpenes and Diterpenoids.Int J Mol Sci. 2019 May 28;20(11):2627. doi: 10.3390/ijms20112627. Int J Mol Sci. 2019. PMID: 31142039 Free PMC article. Review.
-
The isolation and synthesis of neodolastane diterpenoids.Nat Prod Rep. 2015 Feb;32(2):230-55. doi: 10.1039/c4np00077c. Nat Prod Rep. 2015. PMID: 25366359 Review.
Cited by
-
Diterpenes Specially Produced by Fungi: Structures, Biological Activities, and Biosynthesis (2010-2020).J Fungi (Basel). 2022 Feb 28;8(3):244. doi: 10.3390/jof8030244. J Fungi (Basel). 2022. PMID: 35330246 Free PMC article. Review.
-
Catalytic Cyclopropanol Ring Opening for Divergent Syntheses of γ-Butyrolactones and δ-Ketoesters Containing All-Carbon Quaternary Centers.ACS Catal. 2018 Jul 6;8(7):5907-5914. doi: 10.1021/acscatal.8b00711. Epub 2018 May 11. ACS Catal. 2018. PMID: 34457375 Free PMC article.
-
Hyperconjugative Interactions of the Carbon-Halogen Bond that Influence the Geometry of Cyclic α-Haloacetals.J Org Chem. 2022 Apr 15;87(8):5315-5327. doi: 10.1021/acs.joc.2c00148. Epub 2022 Apr 1. J Org Chem. 2022. PMID: 35363473 Free PMC article.
References
-
- Ayer WA, Taube H. Tetrahedron Lett. 1972;13:1917.
- Ayer WA, Taube H. Can. J. Chem. 1973;51:3842.
- Ayer WA, Nakashima TT, Ward DE. Can. J. Chem. 1978;56:2197.
- Allbutt AD, Ayer WA, Brodie HJ, Johri BN, Taube H. Can. J. Microbiol. 1971;17:1401. - PubMed
-
- Ayer WA, Lee SP. Can. J. Chem. 1979;57:3332.
-
-
See inter alia: Kawagishi H, Shimada A, Shirai R, Okamoto K, Ojima F, Sakamoto H, Ishiguro Y, Furukawa S. Tetrahedron Lett. 1994;35:1569. Kita T, Takaya Y, Oshima Y, Ohta T, Aizawa K, Hirano T, Inakuma T. Tetrahedron. 1998;54:11877. Obara Y, Kobayashi H, Ohta T, Ohizumi Y, Nakahata N. Mol. Pharmacol. 2001;59:1287. Obara Y, Hoshino T, Marcotullio MC, Pagiotti R, Nakahata N. Life Sci. 2007;80:1669.
-
-
-
allocyathin B2 and erinacine A: Snider BB, Vo NH, O’Neil SV, Foxman BM. J. Am. Chem. Soc. 1996;118:7644. Snider BB, Vo NH, O’Neil SV. J. Org. Chem. 1998;63:4732. Tori M, Toyoda N, Sono M. J. Org. Chem. 1998;63:306. Takano M, Umino A, Nakada M. Org. Lett. 2004;6:4897. Trost BM, Dong L, Schroeder GM. J. Am. Chem. Soc. 2005;127:2844. Trost BM, Dong L, Schroeder GM. J. Am. Chem. Soc. 2005;127:10259.
-
-
-
sarcodonin G: Piers E, Gilbert M, Cook KL. Org. Lett. 2000;2:1407.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical