Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy
- PMID: 19544395
- PMCID: PMC2814838
- DOI: 10.1002/glia.20900
Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy
Abstract
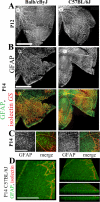
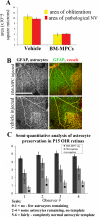
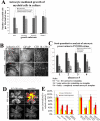
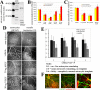
Astrocytes are well known modulators of normal developmental retinal vascularization. However, relatively little is known about the role of glial cells during pathological retinal neovascularization (NV), a leading contributor to vision loss in industrialized nations. We demonstrate that the loss of astrocytes and microglia directly correlates with the development of pathological NV in a mouse model of oxygen-induced retinopathy (OIR). These two distinct glial cell populations were found to have cooperative survival effects in vitro and in vivo. The intravitreal injection of myeloid progenitor cells, astrocytes, or astrocyte-conditioned media rescued endogenous astrocytes from degeneration that normally occurs within the hypoxic, vaso-obliterated retina following return to normoxia. Protection of the retinal astrocytes and microglia was directly correlated with accelerated revascularization of the normal retinal plexuses and reduction of pathological intravitreal NV normally associated with OIR. Using astrocyte-conditioned media, several factors were identified that may contribute to the observed astrocytic protection and subsequent normalization of the retinal vasculature, including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Injection of VEGF or bFGF at specific doses rescued the retinas from developing OIR-associated pathology, an effect that was also preceded by protection of endogenous glia from hypoxia-induced degeneration. Together, these data suggest that vascular-associated glia are also required for normalized revascularization of the hypoxic retina. Methods developed to target and protect glial cells may provide a novel strategy by which normalized revascularization can be promoted and the consequences of abnormal NV in retinal vascular diseases can be prevented.
(c) 2009 Wiley-Liss, Inc.
Figures

References
-
- Adamis AP, Aiello LP, D'Amato RA. Angiogenesis and ophthalmic disease. Angiogenesis. 1999;3(1):9–14. - PubMed
-
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457–61. - PMC - PubMed
-
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. e5. - PubMed
-
- Banin E, Dorrell MI, Aguilar E, Ritter MR, Aderman CM, Smith AC, Friedlander J, Friedlander M. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest Ophthalmol Vis Sci. 2006;47(5):2125–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

