Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways
- PMID: 19544448
- PMCID: PMC2771103
- DOI: 10.1002/stem.96
Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways
Abstract
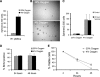
Neural stem cells (NSCs) can be derived from single mouse embryonic stem cells (ESCs) in the absence of instructive factors. Clonal primitive NSC (pNSC) colonies are formed first, and then give rise to clonal, fibroblast growth factor-dependent definitive neural stem cells (dNSCs). We tested low-oxygen culture as a potential method of alleviating the extensive cell death seen in pNSCs and dNSCs. Culture in low (4%) oxygen promoted survival of pNSCs by inhibiting apoptosis-inducing factor (AIF)-dependent cell death, although pNSCs undergo both AIF- and caspase-mediated cell death in 20% oxygen. In contrast, survival of dNSCs in low oxygen was increased by inhibition of caspase-dependent cell death. In normoxia, AIF is implicated in promoting dNSC survival. Neither survival effect was dependent on the main transcriptional effector of hypoxia, hypoxia-inducible factor 1. Low-oxygen concentrations may be involved in expansion of early NSC populations by inhibiting cell death through different pathways in these sequential pNSC and dNSC populations.
Figures


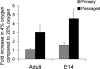

References
-
- Tropepe V, Hitoshi S, Sirard C, et al. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. - PubMed
-
- Csete M. Oxygen in the cultivation of stem cells. Ann Ny Acad Sci. 2005;1049:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

