Role of T cell TGF-beta signaling in intestinal cytokine responses and helminthic immune modulation
- PMID: 19544487
- PMCID: PMC2882993
- DOI: 10.1002/eji.200838956
Role of T cell TGF-beta signaling in intestinal cytokine responses and helminthic immune modulation
Abstract
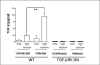
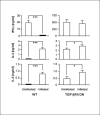
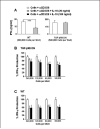
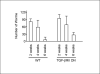
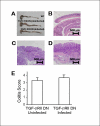
Colonization with helminthic parasites induces mucosal regulatory cytokines, like IL-10 or TGF-beta, that are important in suppressing colitis. Helminths induce mucosal T cell IL-10 secretion and regulate lamina propria mononuclear cell (LPMC) Th1 cytokine generation in an IL-10-dependent manner in WT mice. Helminths also stimulate mucosal TGF-beta release. As TGF-beta exerts major regulatory effects on T lymphocytes, we investigated the role of T lymphocyte TGF-beta signaling in helminthic modulation of intestinal immunity. T cell TGF-beta signaling is interrupted in TGF-beta receptor II dominant negative (TGF-betaRII DN) mice by T-cell-specific over-expression of a TGF-betaRII DN. We studied LPMC responses in WT and TGF-betaRII DN mice that were uninfected or colonized with the nematode, Heligmosomoides polygyrus. Our results indicate an essential role of T cell TGF-beta signaling in limiting mucosal Th1 and Th2 responses. Furthermore, we demonstrate that helminthic induction of intestinal T cell IL-10 secretion requires intact T cell TGF-beta-signaling pathway. Helminths fail to curtail robust, dysregulated intestinal Th1 cytokine production and chronic colitis in TGF-betaRII DN mice. Thus, T cell TGF-beta signaling is essential for helminthic stimulation of mucosal IL-10 production, helminthic modulation of intestinal IFN-gamma generation and H. polygyrus-mediated suppression of chronic colitis.
Figures


Similar articles
-
Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway.J Exp Med. 2010 Oct 25;207(11):2331-41. doi: 10.1084/jem.20101074. Epub 2010 Sep 27. J Exp Med. 2010. PMID: 20876311 Free PMC article.
-
Cutting edge: in the absence of TGF-β signaling in T cells, fewer CD103+ regulatory T cells develop, but exuberant IFN-γ production renders mice more susceptible to helminth infection.J Immunol. 2012 Aug 1;189(3):1113-7. doi: 10.4049/jimmunol.1200991. Epub 2012 Jun 29. J Immunol. 2012. PMID: 22753928 Free PMC article.
-
Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine.Infect Immun. 2007 Sep;75(9):4655-63. doi: 10.1128/IAI.00358-07. Epub 2007 Jul 2. Infect Immun. 2007. PMID: 17606601 Free PMC article.
-
Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation.Immunol Today. 1997 Feb;18(2):61-4. doi: 10.1016/s0167-5699(97)01000-1. Immunol Today. 1997. PMID: 9057354 Review.
-
[Regulation of the immune response to intestinal nematode infections in mice].Wiad Parazytol. 2000;46(4):421-31. Wiad Parazytol. 2000. PMID: 16886322 Review. Polish.
Cited by
-
Regulatory T cells in infection.Adv Immunol. 2011;112:73-136. doi: 10.1016/B978-0-12-387827-4.00003-6. Adv Immunol. 2011. PMID: 22118407 Free PMC article. Review.
-
Characterization of a rhodanese homologue from Haemonchus contortus and its immune-modulatory effects on goat immune cells in vitro.Parasit Vectors. 2020 Sep 7;13(1):454. doi: 10.1186/s13071-020-04333-6. Parasit Vectors. 2020. PMID: 32894178 Free PMC article.
-
The Impact of Cell-Intrinsic STAT6 Protein on Donor T Cell-Mediated Graft-Versus-Tumor Effect.Int J Mol Sci. 2024 Dec 31;26(1):280. doi: 10.3390/ijms26010280. Int J Mol Sci. 2024. PMID: 39796136 Free PMC article.
-
Helminth infections and host immune regulation.Clin Microbiol Rev. 2012 Oct;25(4):585-608. doi: 10.1128/CMR.05040-11. Clin Microbiol Rev. 2012. PMID: 23034321 Free PMC article. Review.
-
Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway.J Exp Med. 2010 Oct 25;207(11):2331-41. doi: 10.1084/jem.20101074. Epub 2010 Sep 27. J Exp Med. 2010. PMID: 20876311 Free PMC article.
References
-
- Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int.J.Parasitol. 2007;37:457–464. - PubMed
-
- Summers RW, Elliott DE, Urban JF, Jr., Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. - PubMed
-
- Finkelman FD, Urban JF., Jr. The other side of the coin: the protective role of the TH2 cytokines. J.Allergy Clin.Immunol. 2001;107:772–780. - PubMed
-
- Sacco R, Hagen M, Sandor M, Weinstock JV, Lynch RG. Established T(H1) granulomatous responses induced by active Mycobacterium avium infection switch to T(H2) following challenge with Schistosoma mansoni. Clin.Immunol. 2002;104:274–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK034928/DK/NIDDK NIH HHS/United States
- DK07663/DK/NIDDK NIH HHS/United States
- DK034928/DK/NIDDK NIH HHS/United States
- R01 DK038327/DK/NIDDK NIH HHS/United States
- T32 DK007663/DK/NIDDK NIH HHS/United States
- T32 AI007511/AI/NIAID NIH HHS/United States
- R01 DK076638/DK/NIDDK NIH HHS/United States
- R56 DK058755/DK/NIDDK NIH HHS/United States
- DK58755/DK/NIDDK NIH HHS/United States
- P30 DK025295/DK/NIDDK NIH HHS/United States
- DK25295/DK/NIDDK NIH HHS/United States
- DK38327/DK/NIDDK NIH HHS/United States
- R01 DK058755/DK/NIDDK NIH HHS/United States
- T32AI007511/AI/NIAID NIH HHS/United States
- K08 DK082913/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

