Multicomponent supramolecular systems: self-organization in coordination-driven self-assembly
- PMID: 19544512
- PMCID: PMC2765196
- DOI: 10.1002/chem.200900230
Multicomponent supramolecular systems: self-organization in coordination-driven self-assembly
Abstract
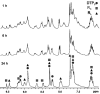
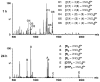
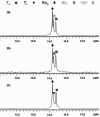
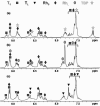
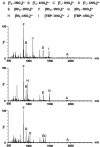
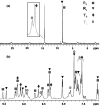
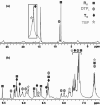
The self-organization of multicomponent supramolecular systems involving a variety of two-dimensional (2 D) polygons and three-dimensional (3 D) cages is presented. Nine self-organizing systems, SS(1)-SS(9), have been studied. Each involves the simultaneous mixing of organoplatinum acceptors and pyridyl donors of varying geometry and their selective self-assembly into three to four specific 2 D (rectangular, triangular, and rhomboid) and/or 3 D (triangular prism and distorted and nondistorted trigonal bipyramidal) supramolecules. The formation of these discrete structures is characterized using NMR spectroscopy and electrospray ionization mass spectrometry (ESI-MS). In all cases, the self-organization process is directed by: 1) the geometric information encoded within the molecular subunits and 2) a thermodynamically driven dynamic self-correction process. The result is the selective self-assembly of multiple discrete products from a randomly formed complex. The influence of key experimental variables--temperature and solvent--on the self-correction process and the fidelity of the resulting self-organization systems is also described.
Figures
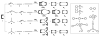
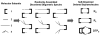
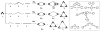







Similar articles
-
Size selective self-sorting in coordination-driven self-assembly of finite ensembles.Inorg Chem. 2008 Jun 2;47(11):4706-11. doi: 10.1021/ic800038j. Epub 2008 Apr 23. Inorg Chem. 2008. PMID: 18433099
-
Self-organization in coordination-driven self-assembly.Acc Chem Res. 2009 Oct 20;42(10):1554-63. doi: 10.1021/ar900077c. Acc Chem Res. 2009. PMID: 19555073 Free PMC article. Review.
-
A facile approach toward multicomponent supramolecular structures: selective self-assembly via charge separation.J Am Chem Soc. 2010 Dec 1;132(47):16873-82. doi: 10.1021/ja106251f. Epub 2010 Nov 5. J Am Chem Soc. 2010. PMID: 21053935 Free PMC article.
-
Self-recognition in the coordination driven self-assembly of 2-D polygons.Inorg Chem. 2004 Aug 23;43(17):5335-8. doi: 10.1021/ic049326p. Inorg Chem. 2004. PMID: 15310211
-
Self-Assembly Methods for Recently Reported Discrete Supramolecular Structures Based on Terpyridine.Chem Asian J. 2021 Dec 13;16(24):4037-4048. doi: 10.1002/asia.202101136. Epub 2021 Oct 29. Chem Asian J. 2021. PMID: 34672098 Review.
Cited by
-
Inducing Social Self-Sorting in Organic Cages To Tune The Shape of The Internal Cavity.Angew Chem Int Ed Engl. 2020 Sep 14;59(38):16755-16763. doi: 10.1002/anie.202007571. Epub 2020 Jul 16. Angew Chem Int Ed Engl. 2020. PMID: 32542926 Free PMC article.
-
Triple Self-Sorting in Constitutional Dynamic Networks: Parallel Generation of Imine-Based CuI , FeII , and ZnII Complexes.Angew Chem Int Ed Engl. 2020 Jul 20;59(30):12484-12492. doi: 10.1002/anie.202000818. Epub 2020 Jun 3. Angew Chem Int Ed Engl. 2020. PMID: 32286724 Free PMC article.
-
The self-sorting behavior of circular helicates and molecular knots and links.Angew Chem Int Ed Engl. 2014 Jul 21;53(30):7823-7. doi: 10.1002/anie.201404270. Epub 2014 Jun 4. Angew Chem Int Ed Engl. 2014. PMID: 24899408 Free PMC article.
-
Construction of hexagonal prisms of variable size via coordination-driven multicomponent self-assembly.Inorg Chem. 2010 Oct 4;49(19):8653-5. doi: 10.1021/ic1014219. Inorg Chem. 2010. PMID: 20809652 Free PMC article.
-
Self-assembled supramolecular hetero-bimetallacycles for anticancer potency by intracellular release.Chemistry. 2014 Oct 27;20(44):14410-20. doi: 10.1002/chem.201403372. Epub 2014 Sep 10. Chemistry. 2014. PMID: 25209962 Free PMC article.
References
-
- Lehn J-M. Angew. Chem. Int. Ed.. Engl. 1988;27:89–112.
- Cram DJ. Angew. Chem. Int. Ed.. Engl. 1988;27:1009–1020.
- Pederson CJ. Angew. Chem. Int. Ed.. Engl. 1988;27:1021–1027.
-
- Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001:509–518.
- Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew. Chem. Int. Ed. 2004;43:3644. - PubMed
- Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:349. - PubMed
- Gianneschi NC, Masar MS, III, Mirkin CA. Acc. Chem. Res. 2005;38:825. - PubMed
-
- Prins LJ, Reinhoudt DN, Timmerman P. Angew. Chem. Int. Ed. 2001;40:2382–2426. - PubMed
- Corbin PS, Lawless LJ, Li Z, Ma Y, Witmer MJ, Zimmerman SC. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5099–5104. - PMC - PubMed
- B. Purse W, Rebek J., Jr. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10777–10782. - PMC - PubMed
-
- Pollino JM, Weck M. Chem. Soc. Rev. 2005;34:193–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

