Inhaled house dust mite induces pulmonary T helper 2 cytokine production
- PMID: 19545261
- PMCID: PMC3385347
- DOI: 10.1111/j.1365-2222.2009.03302.x
Inhaled house dust mite induces pulmonary T helper 2 cytokine production
Abstract
Background: Inhaled house dust mite (HDM) results in T-helper (TH) 2 type pathology in unsensitized mice, in conjunction with airway hyperreactivity and airway remodelling. However, the pulmonary cytokine and chemokine profile has not been reported.
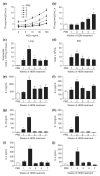
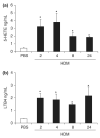
Methods: We have performed a time course analysis of the characteristic molecular mediators and cellular influx in the bronchoalveolar lavage (BAL) and lung in order to define the pulmonary inflammatory response to inhaled HDM extract. Mice were exposed five times a week to soluble HDM extract for 3 weeks. Lung function was measured in groups of mice at intervals following the final HDM challenge. Recruitment of inflammatory cells and inflammatory mediator production was then assessed in BAL and lungs of individual mice.
Results: We found that Th2 cytokines were significantly increased in BAL and lung after HDM challenge from as early as 2 h post-final challenge. The levels of cytokines and chemokines correlated with the influx of eosinophils and Th2 cells to the different compartments of the lung. However, the production of key cytokines such as IL-4, IL-5 and IL-13 preceded the increase in airways resistance.
Conclusion: Inhaled HDM challenge induces a classical Th2 inflammatory mediator profile in the BAL and lung. These data are important for studies determining the efficacy of novel treatment strategies for allergic airways disease.
Figures






References
-
- Kay AB. T cells as orchestrators of the asthmatic response. Ciba Found Symp. 1997;206:56–67. - PubMed
-
- Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–15. - PubMed
-
- Borish LC, Nelson HS, Lanz MJ, et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999;160:1816–23. - PubMed
-
- Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

